
酸碱平衡及紊乱Acid - Base Balanceand DisturbancesPathophysiology Department, ShiHeZi Medical College,HUST
酸碱平衡及紊乱 Acid – Base Balance and Disturbances Pathophysiology Department, ShiHeZi Medical College, HUST
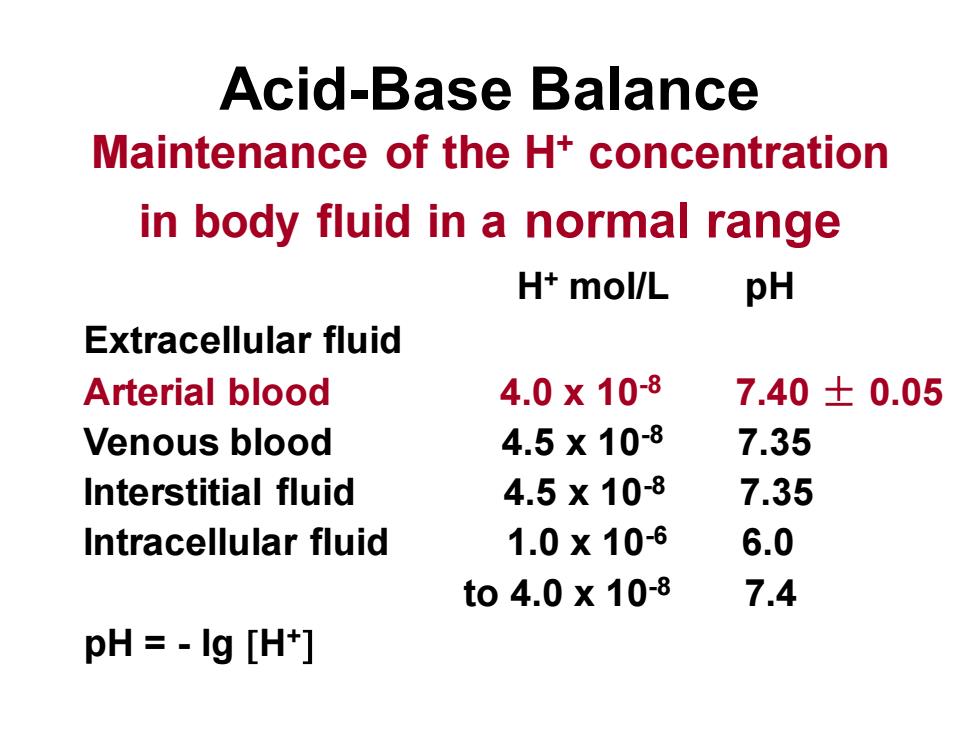
Acid-Base BalanceMaintenance of the H+ concentrationin body fluid in a normal rangepHH+ mol/LExtracellular fluid4.0 x 10-8Arterialblood7.40 ± 0.054.5 x 10-87.35Venous blood4.5 x 10-87.35Interstitial fluid1.0 x 10-66.0Intracellular fluidto 4.0 x 10-87.4pH = - Ig [H+]
Acid-Base Balance Maintenance of the H+ concentration in body fluid in a normal range H+ mol/L pH Extracellular fluid Arterial blood 4.0 x 10-8 7.40 ± 0.05 Venous blood 4.5 x 10-8 7.35 Interstitial fluid 4.5 x 10-8 7.35 Intracellular fluid 1.0 x 10-6 6.0 to 4.0 x 10-8 7.4 pH = - lg H+

Why is the acid - basebalance important for life ?
Why is the acid - base balance important for life ?
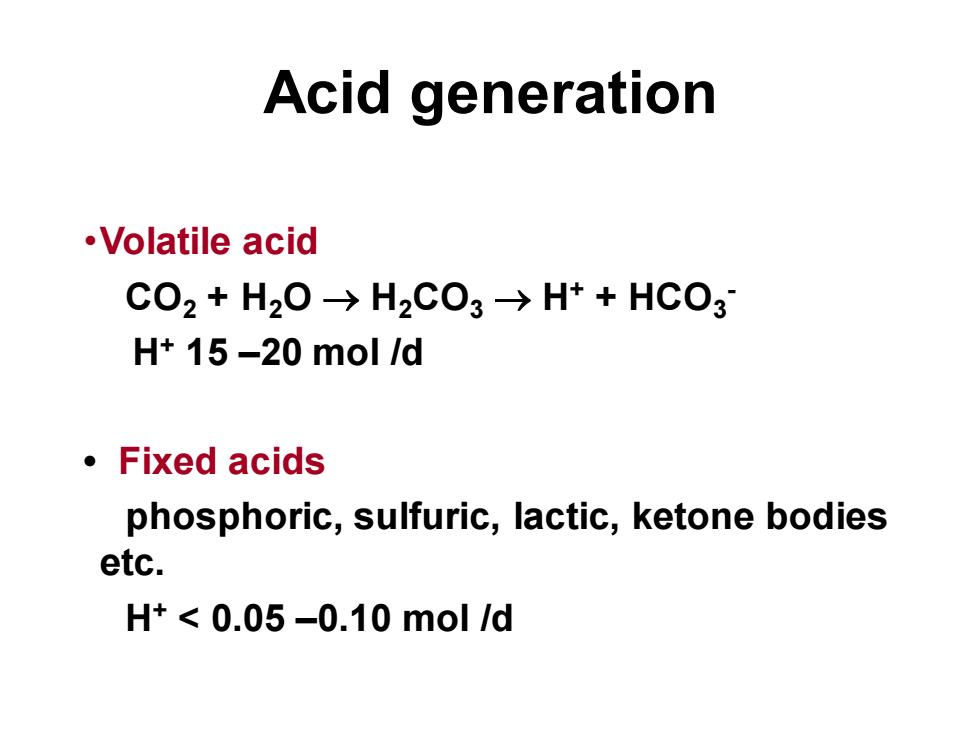
Acid generation·VolatileacidCO2 + H2O →H2CO3 →H+ + HCO3H+ 15 -20 mol /dFixedacidsphosphoric, sulfuric, lactic, ketone bodiesetc.H+ < 0.05 -0.10 mol /d
Acid generation •Volatile acid CO2 + H2O → H2CO3 → H+ + HCO3 - H+ 15 –20 mol /d • Fixed acids phosphoric, sulfuric, lactic, ketone bodies etc. H+ < 0.05 –0.10 mol /d
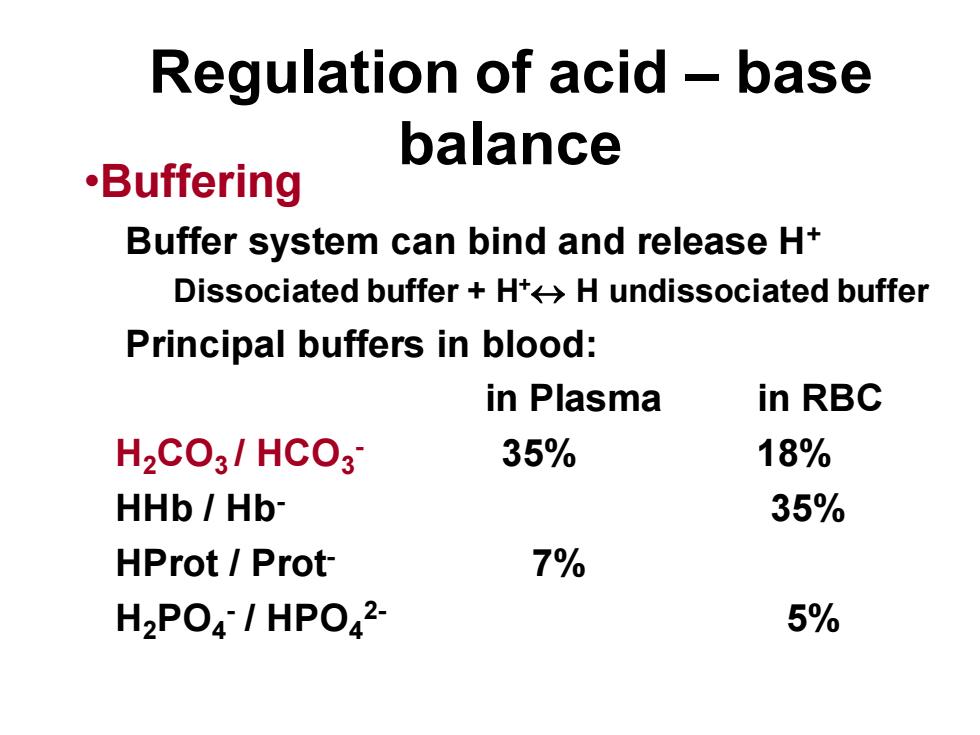
Regulation of acid - basebalance·BufferingBuffer system can bind and release H+Dissociatedbuffer+HtHundissociatedbufferPrincipal buffers in blood:in RBCin Plasma35%18%H2CO3/ HCO335%HHb / Hb*7%HProt / Prot5%H2PO4- / HPO.2
Regulation of acid – base balance •Buffering Buffer system can bind and release H+ Dissociated buffer + H+ H undissociated buffer Principal buffers in blood: in Plasma in RBC H2CO3 / HCO3 - 35% 18% HHb / Hb- 35% HProt / Prot- 7% H2PO4 - / HPO4 2- 5%