
7.在纯化过程中酶活力的损失 主要原因:蛋白质变性 避免方法:改变温度和pH 其他原因:辅助因子被去除
7.在纯化过程中酶活力的损失 主要原因:蛋白质变性 避免方法:改变温度和pH 其他原因:辅助因子被去除
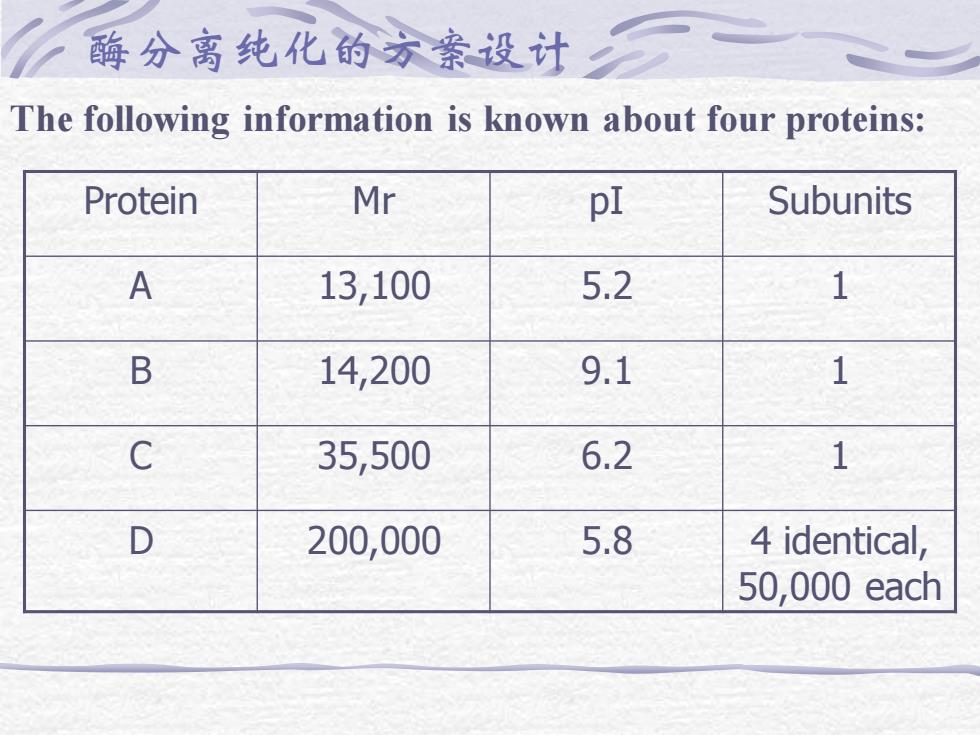
酶分离纯化的方案设计 The following information is known about four proteins: Protein Mr pI Subunits A 13,100 5.2 1 B 14,200 9.1 1 C 35,500 6.2 1 D 200,000 5.8 4 identical, 50,000 each
酶分离纯化的方案设计 The following information is known about four proteins: Protein Mr pI Subunits A 13,100 5.2 1 B 14,200 9.1 1 C 35,500 6.2 1 D 200,000 5.8 4 identical, 50,000 each

a. Sketch the elution profile expected if this mixture is run on a Sephadex G-100 gel filtration column run in 50 mM phosphate buffer, pH 6. Label the peaks. b. Sketch the gel obtained from running these proteins on an SDS polyacrylamide electrophoresis gel at pH 8.0, (after staining and destaining). c. What predictions can you make about the results of a native PAGE at pH 7.6 ( state any assumptions you might need to make about the % acrylamide in the gel )
a. Sketch the elution profile expected if this mixture is run on a Sephadex G-100 gel filtration column run in 50 mM phosphate buffer, pH 6. Label the peaks. b. Sketch the gel obtained from running these proteins on an SDS polyacrylamide electrophoresis gel at pH 8.0, (after staining and destaining). c. What predictions can you make about the results of a native PAGE at pH 7.6 ( state any assumptions you might need to make about the % acrylamide in the gel )

d. Sketch the elution profile of these proteins from a carboxymethyl cellulose ion exchange chromatography column, run at pH 6.25 (with a salt gradient, if necessary). Label the peaks
d. Sketch the elution profile of these proteins from a carboxymethyl cellulose ion exchange chromatography column, run at pH 6.25 (with a salt gradient, if necessary). Label the peaks

第七节 蛋白质浓度的测定 凯氏定氮法 双缩脲法 福林(Folin)-酚试剂法 考马斯蓝染色法 紫外光吸收法
第七节 蛋白质浓度的测定 凯氏定氮法 双缩脲法 福林(Folin)-酚试剂法 考马斯蓝染色法 紫外光吸收法