
率5%106,1。但长期疗效欠佳,2年内几平均有复发或转移。主要副作用是骨髓抑制。核素 标记的奥曲肽可用于MBG阴性者,但疗效尚难评价。 3.放疗和化疗外放射治疗推荐于无法手术切除的肿痘和缓解骨转移所致疼痛,但可 能加重高血压101。化疗推荐CVD方案(环磷酰胶、长春新碱、氯烯唑胺),有效率约50%, 但多于2年内复发9,1H0。联合MBG可能提高疗效1。抗血管生成靶向药物治疗可能有效 02,142-116。 4.处理儿茶酚胺增多症对于恶性或因故不能手术者推荐α-受体阻滞剂、B受体阻滞剂 控制高血压。 六、预后和随访 (一)预后 PHEO/PGL的预后与年龄、良恶性、有无家族史及治疗早晚等有关。良性者5年生存率 >95%,但约50%志者仍持续高血压。复发率为6.5%一17%,复发者恶性率约50%,家族 性、肾上腺外及右侧者更易复发。恶性PHE0/PGL不可治愈,5年生存率约50%1叫,肝、 肺转移较骨转移者预后差46,1021,其中约50%死于1~3年,但约50%可存活20年以上4。 (二)随访 1.随访原因 (1)肿瘤有无残留: (2)病理难于鉴别良恶性,主要依据其临床出现转移: (3)易复发、多发,特别是家族发病者。 2.随访内容:包括临床症状(如高血压)、生化指标(如血浆游离MNS、24h尿CA和 分馏的MNS)、CT扫描等。 3.随访方案 (1)推荐术后10~14天复查血尿生化指标6,11,判断肿瘤是否残留、有无转移等。 (2)敢发病例单侧肾上腺切除者每年一次,至少连续10年1刀。 (3)高危群体(SDHB突变、PGL、肿瘤体积巨大)和遗传性PHEO/PGL者每6~12个月 复查1次临床和生化指标,终生随访1
16 率5%[106, 107]。但长期疗效欠佳,2年内几乎均有复发或转移。主要副作用是骨髓抑制。核素 标记的奥曲肽可用于MIBG阴性者,但疗效尚难评价。 3.放疗和化疗 外放射治疗推荐于无法手术切除的肿瘤和缓解骨转移所致疼痛,但可 能加重高血压[108]。化疗推荐 CVD 方案(环磷酰胺、长春新碱、氮烯唑胺),有效率约50%, 但多于2年内复发[109, 110]。联合MIBG可能提高疗效[111]。抗血管生成靶向药物治疗可能有效 [102, 112-116]。 4. 处理儿茶酚胺增多症 对于恶性或因故不能手术者推荐α-受体阻滞剂、β-受体阻滞剂 控制高血压[8]。 六、预后和随访 (一)预后 PHEO/PGL的预后与年龄、良恶性、有无家族史及治疗早晚等有关。良性者5年生存率 >95%,但约50%患者仍持续高血压[117]。复发率为6.5%~17%,复发者恶性率约50%,家族 性、肾上腺外及右侧者更易复发[6]。恶性PHEO/PGL不可治愈,5年生存率约 50%[102],肝、 肺转移较骨转移者预后差[46, 102],其中约50%死于1~3年,但约50%可存活20年以上[84]。 (二)随访 1.随访原因 (1)肿瘤有无残留; (2)病理难于鉴别良恶性,主要依据其临床出现转移; (3)易复发、多发,特别是家族发病者。 2.随访内容:包括临床症状(如高血压)、生化指标(如血浆游离MNs、24h尿CA和 分馏的MNs)、CT扫描等。 3.随访方案 (1)推荐术后10~14天复查血尿生化指标[36, 118],判断肿瘤是否残留、有无转移等。 (2)散发病例单侧肾上腺切除者每年一次,至少连续10年[117]。 (3)高危群体(SDHB突变、PGL、肿瘤体积巨大)和遗传性PHEO/PGL者每6~12个月 复查1次临床和生化指标,终生随访[119]
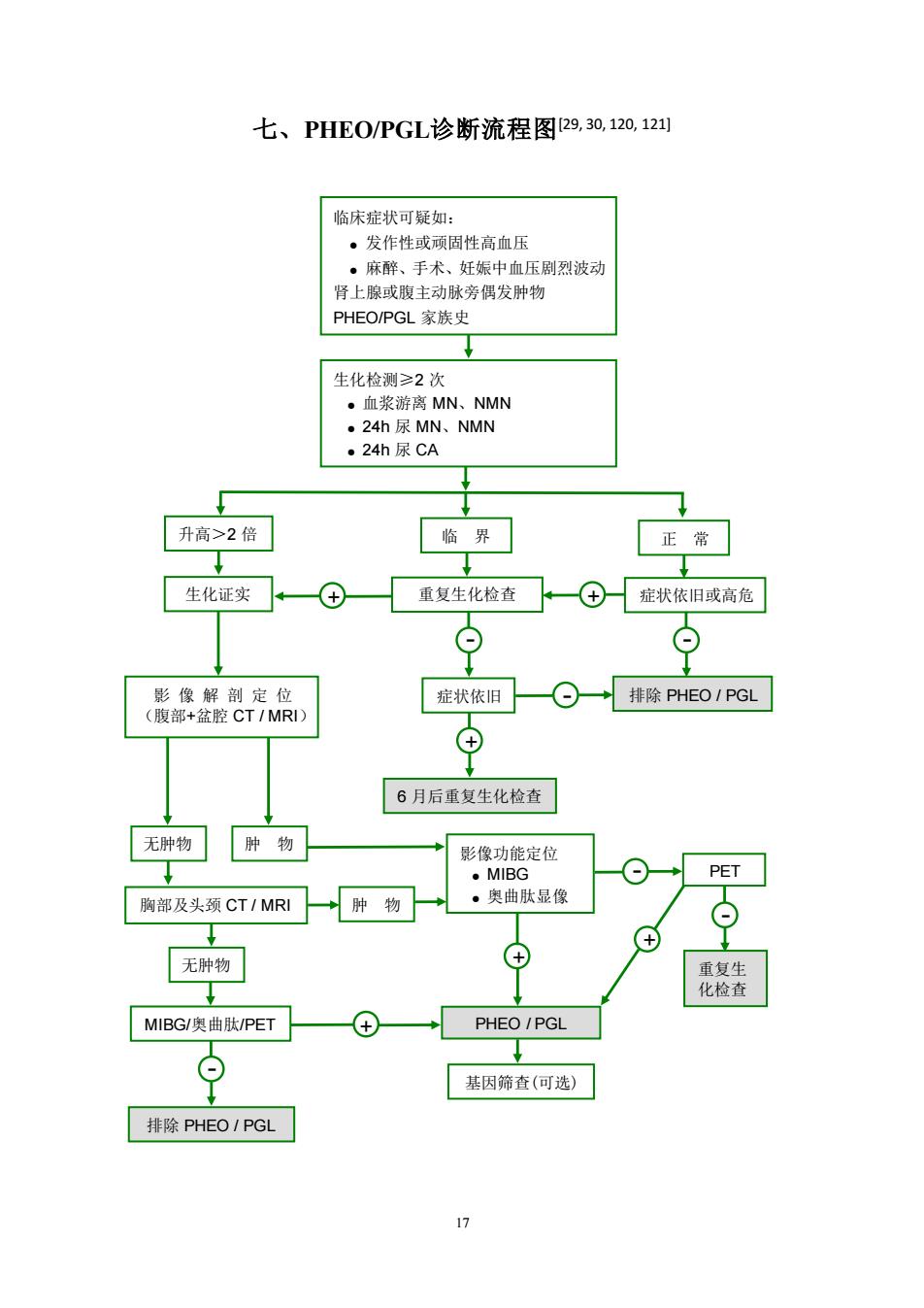
七、PHE0/PGL诊断流程图29,30,120,12 临床症状可疑如 ·发作性或顾固性高血压 。麻醉、手术、妊娠中血压剧烈波动 肾上腺或腹主动脉旁偶发肿物 PHEO/PGL家族史 生化检测≥2次 。血浆游离MN、NMN ·24h尿MN、NMN ·24h尿CA 升高>2倍 临界 正常 生化证实 重复生化检查、—④症状依旧或高危 质品 症状依旧一⊙一→排除PHEO1PGL ⊕ 6月后重复生化检查 无肿物肿物 影像功能定位 ·MIBG PET 胸部及头颈CT/MRI →肿物 ·奥曲肽显像 ⊙ 无肿物 MIBG/奥曲肽/PET PHEO/PGL 基因筛查(可选) 排除PHEO1PGL
17 七、PHEO/PGL诊断流程图[29, 30, 120, 121] 无肿物 肿 物 胸部及头颈 CT / MRI 无肿物 肿 物 排除 PHEO / PGL MIBG/奥曲肽/PET - + 影像功能定位 ● MIBG ● 奥曲肽显像 + PHEO / PGL 基因筛查(可选) + - PET - 重复生 化检查 临床症状可疑如: ● 发作性或顽固性高血压 ● 麻醉、手术、妊娠中血压剧烈波动 肾上腺或腹主动脉旁偶发肿物 PHEO/PGL 家族史 生化检测≥2 次 ● 血浆游离 MN、NMN ● 24h 尿 MN、NMN ● 24h 尿 CA 升高>2 倍 临 界 正 常 生化证实 + 重复生化检查 + 症状依旧或高危 - - 影 像 解 剖 定 位 (腹部+盆腔 CT / MRI) 6 月后重复生化检查 症状依旧 - 排除 PHEO / PGL +

八、PHE0PGL治疗流程图29,0,110,12四 嗜铬细胞痴/副神经节瘤(PHEO/PGL) 药物准备 ·酚苄明 。哌唑嗪、多沙唑嗪 ·钙离子洁抗剂 。高血压危象:硝普钠、乌拉地尔 ·心律失常:B.受体阻滞剂 性 肾上腺 上腺外 原发局限病灶 转移病灶 能否“根治”切除? 可切除 不可切除 散发性遗传性 原发灶+ 移灶切除 肿瘤切除 ·3-MIBG ·化 131-MIBG 。放疗 随访≥10年 终生随访 (a)MIBG昆像阳性者 (b)奥曲肽显像阳性者
18 八、PHEO/PGL治疗流程图[29, 30, 110, 122] 嗜铬细胞瘤/副神经节瘤(PHEO/PGL) 药物准备 ● 酚苄明 ● 哌唑嗪、多沙唑嗪 ● 钙离子拮抗剂 ● 高血压危象:硝普钠、乌拉地尔 ● 心律失常:β-受体阻滞剂 良 性 恶 性 肾上腺 肾上腺外 原发局限病灶 转移病灶 单 侧 双 侧 能否“根治”切除? + - 可切除 不可切除 散发性 遗传性 原发灶+转 移灶切除 减瘤 手术 肿瘤切除 保留肾上腺 的肿瘤切除 肿瘤 切除 “根治” 切除 减瘤 切除 ● 131I-MIBG (a) ● 奥曲肽 (b) ● 化疗 131I-MIBG (a) ● 放疗 随访≥10 年 终生随访 (a)MIBG 显像阳性者 (b)奥曲肽显像阳性者

参考文献 M. elli M.et a Phaeochro 28 26 es.-ic et The 2 eoeof neromast peepe 阁 阁 prospects.Endocr Rev. Agarwal SC,et al Phacochro ytomas presenting as acute rises afer beta 【18 E.Da adNExra-adrenal Nephrol,( f unusual locations of extra-adrenal 05.1s8592 22 23 24 19.0 H Endocrinol Diabetes 26 291 2331-9 Karag iann B phe 潘东亮,李汉忠,曾正略,咯铬细胞瘤临床功能分级与术前准名标准的深讨:中华外科杂志 M m 200). omocytoma. B
19 参考文献 [1] DeLellis RA LRV, Heitz PU ea. Pathology and genetics of tumours of endocrine organs. World Health Organization classification of tumors :Lyon: IARC Press,2004. [2] Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract, 2000,6(3):249-52. [3] Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res, 2004,27(3):193-202. [4] Pederson LC, Lee JE. Pheochromocytoma. Curr Treat Options Oncol, 2003,4(4):329-37. [5] Fung MM, Viveros OH, O'Connor DT. Diseases of the adrenal medulla. Acta Physiol (Oxf), 2008,192(2):325-35. [6] Amar L, Servais A, Gimenez-Roqueplo AP, et al. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab, 2005,90(4):2110-6. [7] Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential di agnosis. J Natl Cancer Inst, 2003,95(16):1196-204. [8] Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet, 2005,366(9486):665-75. [9] Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol, 2005,23(34):8812-8. [10] Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med, 2002,346(19):1459-66. [11] Barontini M, Levin G, Sanso G. Characteristics of pheochromocytoma in a 4- to 20-year-old population. Ann N Y Acad Sci, 2006,1073:30-7. [12] Eng C. Seminars in medicine of the Beth Israel Hospital, Boston. The RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med, 1996,335(13):943-51. [13] Maher ER, Kaelin WG Jr. von Hippel-Lindau disease. Medicine (Baltimore), 1997,76(6):381-91. [14] Gutmann DH, Geist RT, Rose K, et al. Loss of neurofibromatosis type I (NF1) gene expression in pheochromocytomas from patients without NF1. Genes Chromosomes Cancer, 1995,13(2):104-9. [15] 张卫东, 李虹等. 特殊类型的嗜铬细胞瘤. 中华泌尿外科杂志. 17,1996. 143. [16] Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev, 2003,24(4):539-53. [17] Sibal L, Jovanovic A, Agarwal SC, et al. Phaeochromocytomas presenting as acute crises after beta blockade therapy. Clin Endocrinol (Oxf), 2006,65(2):186-90. [18] Bravo EL. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocr Rev, 1994,15(3):356-68. [19] Atiyeh BA, Barakat AJ, Abumrad NN. Extra-adrenal pheochromocytoma. J Nephrol, 1997,10(1):25-9. [20] Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol, 1992,147(1):1-10. [21] Sahdev A, Sohaib A, Monson JP, et al. CT and MR imaging of unusual locations of extra-adrenal paragangliomas (pheochromocytomas). Eur Radiol, 2005,15(1):85-92. [22] Khafagi FA, Shapiro B, Fischer M, et al. Phaeochromocytoma and functioning paraganglioma in childhood and adolescence: role of iodine 131 metaiodobenzylguanidine. Eur J Nucl Med, 1991,18(3):191-8. [23] Levine LS&DAM. Pheochromocytoma. In: RE Behrman RMK&HBJ, editor. Nelson Textbook of Pediatrics. 16 th editioned. USA: Saunders:Philadelphia,2000. 1741–1743. [24] Mundschenk J, Lehnert H. Malignant pheochromocytoma. Exp Clin Endocrinol Diabetes, 1998,106(5):373-6. [25] Manger WM, Eisenhofer G. Pheochromocytoma: diagnosis and management update. Curr Hypertens Rep, 2004,6(6):477-84. [26] Manger WM. An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges. Ann N Y Acad Sci, 2006,1073:1-20. [27] Yeo H, Roman S. Pheochromocytoma and functional paraganglioma. Curr Opin Oncol, 2005,17(1):13-8. [28] Zelinka T, Eisenhofer G, Pacak K. Pheochromocytoma as a catecholamine producing tumor: implications for clinical practice. Stress, 2007,10(2):195-203. [29] Reisch N, Peczkowska M, Januszewicz A, et al. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens, 2006,24(12):2331-9. [30] Karagiannis A, Mikhailidis DP, Athyros VG, et al. Pheochromocytoma: an update on genetics and management. Endocr Relat Cancer, 2007,14(4):935-56. [31] Jimenez C, Cote G, Arnold A, et al. Review: Should patients with apparently sporadic pheochromocytomas or paragangliomas be screened for hereditary syndromes. J Clin Endocrinol Metab, 2006,91(8):2851-8. [32] Kuruba R, Gallagher SF. Current management of adrenal tumors. Curr Opin Oncol, 2008,20(1):34-46. [33] 潘东亮, 李汉忠, 曾正陪. 嗜 铬细胞瘤临 床功能分级与 术前准备标 准的探讨. 中 华外科杂志, 2004,(18):4-7. [34] Brouwers FM, Eisenhofer G, Lenders JW, et al. Emergencies caused by pheochromocytoma, neuroblastoma, or ganglioneuroma. Endocrinol Metab Clin North Am, 2006,35(4):699-724, viii. [35] Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med

【361 mnmendationsforciealprartiefiomn B7] B38] Plasma me 401 shofer G.Ky ofpheoch 621750nm t is be (41) 421 w8i山o on of 24-hour urinary 。 【43 G rediscovery as a [45] allo S imaging in characterisation of adrena [4 】 adi02002412113-2 ver 48 years with pheo chromocy tom 47 ithm for the diagr nol Ad 2004,89%2479-.9 combined unenhanced and 149] of dia nd localization tests for )2521- nal tumors [51 K, 08.15090 nzy lguanidine (MIBG) [53 uptake in begnign [54 200 9182 【5 n D. clinical presentation and treatmen [56] Diagnostic ace y of radionuclide imaging using 131 nor-che creting adrena 【58 Berch mer .et al Misleading diagnosis retroperitonea I59咧 13/7513. 【60] 4 61 ning EP.Reubi JC The 62 .etal Pheo mocytomas:detection with 18F DOPA whole body 63] N 05689-94. ission tomography for the detection of I64 EiCWeR92betebssohemhmogomtwtooseamadowNa
20 2007,356(6):601-10. [36] Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab, 2007,3(2):92-102. [37] Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev, 2004,56(3):331-49. [38] Eisenhofer G, Friberg P, Pacak K, et al. Plasma metadrenalines: do they provide useful information about sympatho-adrenal function and catecholamine metabolism. Clin Sci (Lond), 1995,88(5):533-42. [39] Eisenhofer G, Keiser H, Friberg P, et al. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab, 1998,83(6):2175-85. [40] Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best. JAMA, 2002,287(11):1427-34. [41] Sawka AM, Gafni A, Thabane L, et al. The economic implications of three biochemical screening algorithms for pheochromocytoma. J Clin Endocrinol Metab, 2004,89(6):2859-66. [42] Sawka AM, Jaeschke R, Singh RJ, et al. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab, 2003,88(2):553-8. [43] Eisenhofer G, Goldstein DS, Kopin IJ, et al. Pheochromocytoma: rediscovery as a catecholamine-metabolizing tumor. Endocr Pathol, 2003,14(3):193-212. [44] Eisenhofer G, Goldstein DS, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab, 2003,88(6):2656-66. [45] Honigschnabl S, Gallo S, Niederle B, et al. How accurate is MR imaging in characterisation of adrenal masses: update of a long-term study. Eur J Radiol, 2002,41(2):113-22. [46] Goldstein RE, O'Neill JA Jr, 3rd HGW, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg, 1999,229(6):755-64; discussion 764-6. [47] Ilias I, Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab, 2004,89(2):479-91. [48] Caoili EM, Korobkin M, Francis IR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology, 2002,222(3):629-33. [49] Witteles RM, Kaplan EL, Roizen MF. Sensitivity of diagnostic and localization tests for pheochromocytoma in clinical practice. Arch Intern Med, 2000,160(16):2521-4. [50] Lauriero F, Rubini G, D'Addabbo F, et al. I-131 MIBG scintigraphy of neuroectodermal tumors. Comparison between I-131 MIBG and In-111 DTPA-octreotide. Clin Nucl Med, 1995,20(3):243-9. [51] Pacak K, Ilias I, Adams KT, et al. Biochemical diagnosis, localization and management of pheochromocytoma: focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. J Intern Med, 2005,257(1):60-8. [52] Greenblatt DY, Shenker Y, Chen H. The utility of metaiodobenzylguanidine (MIBG) scintigraphy in patients with pheochromocytoma. Ann Surg Oncol, 2008,15(3):900-5. [53] der Harst E v, de Herder WW, Bruining HA, et al. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab, 2001,86(2):685-93. [54] Miskulin J, Shulkin BL, Doherty GM, et al. Is preoperative iodine 123 meta-iodobenzylguanidine scintigraphy routinely necessary before initial adrenalectomy for pheochromocytoma. Surgery, 2003,134(6):918-22; discussion 922-3. [55] Erickson D, Kudva YC, Ebersold MJ, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab, 2001,86(11):5210-6. [56] Ilias I, Yu J, Carrasquillo JA, et al. Superiority of 6-[18F]-fluorodopamine positron emission tomography versus [131I]-metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. J Clin Endocrinol Metab, 2003,88(9):4083-7. [57] Maurea S, Klain M, Caraco C, et al. Diagnostic accuracy of radionuclide imaging using 131I nor-cholesterol or meta-iodobenzylguanidine in patients with hypersecreting or non-hypersecreting adrenal tumours. Nucl Med Commun, 2002,23(10):951-60. [58] Berchtenbreiter C, Bruning R, Auernhammer A, et al. Misleading diagnosis of retroperitoneal actinomycosis. Eur Radiol, 1999,9(9):1869-72. [59] Solanki KK, Bomanji J, Moyes J, et al. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG). Nucl Med Commun, 1992,13(7):513-21. [60] Epelbaum J, Bertherat J, Prevost G, et al. Molecular and pharmacological characterization of somatostatin receptor subtypes in adrenal, extraadrenal, and malignant pheochromocytomas. J Clin Endocrinol Metab, 1995,80(6):1837-44. [61] Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev, 1991,12(4):450-82. [62] Hoegerle S, Nitzsche E, Altehoefer C, et al. Pheochromocytomas: detection with 18F DOPA whole body PET--initial results. Radiology, 2002,222(2):507-12. [63] Hoegerle S, Ghanem N, Altehoefer C, et al. 18F-DOPA positron emission tomography for the detection of glomus tumours. Eur J Nucl Med Mol Imaging, 2003,30(5):689-94. [64] Plouin PF, Gimenez-Roqueplo AP. The genetic basis of pheochromocytoma: who to screen and how. Nat Clin Pract Endocrinol Metab, 2006,2(2):60-1