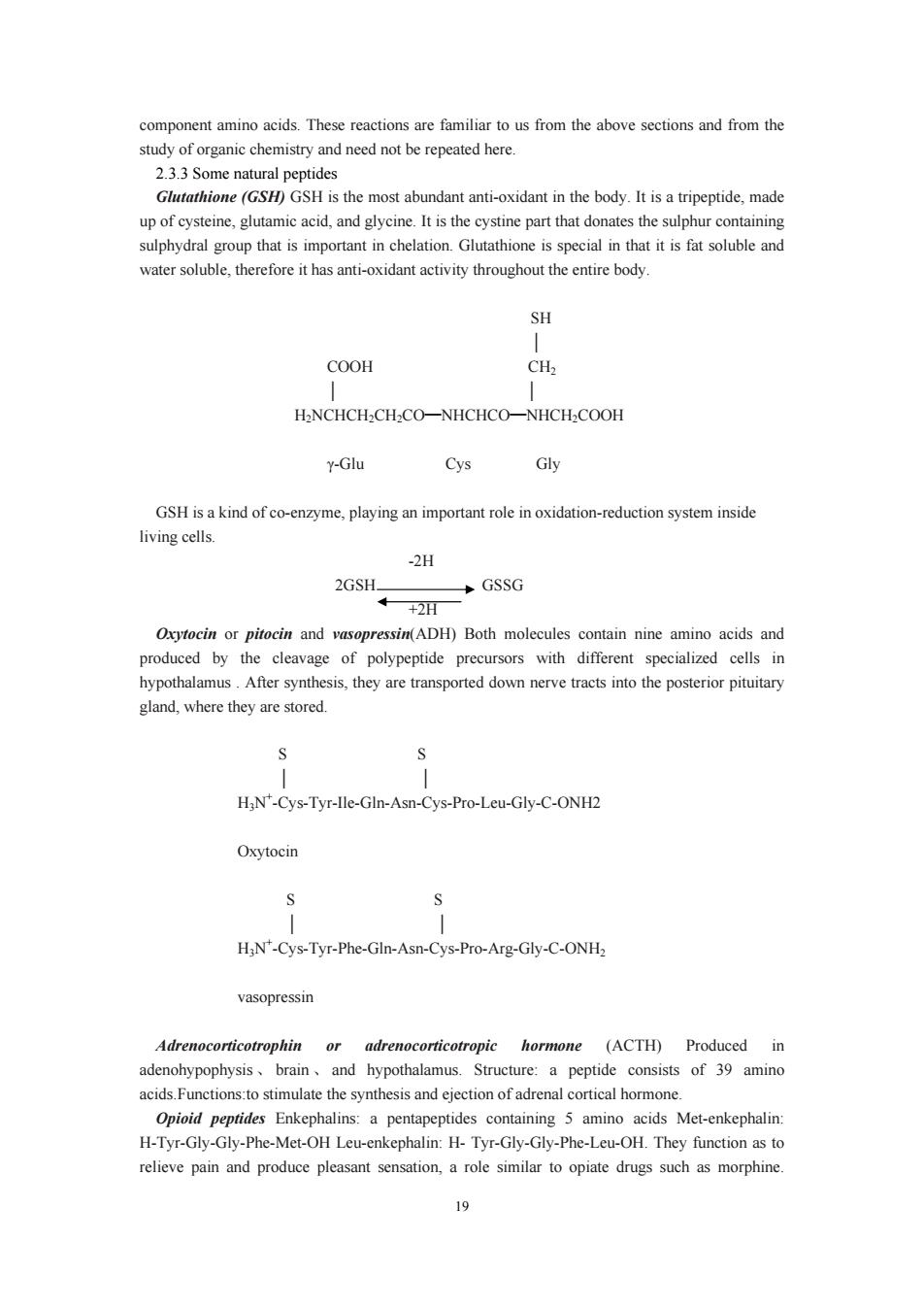
component amino acids.These reactions are familiar to us from the above sections and from the Glutathione (GSH)GSH is the most abundant anti-oxidant in the body.It is a tripeptide,made up of cysteine,glutamic acid,and glycine.It is the cystine part that donates the sulphur containing sulphydral group that is important in chelation.Glutathione is special in that it is fat soluble and water soluble,therefore it has anti-oxidant activity throughout the entire body. SH COOH CH2 H.NCHCH2CH-CO-NHCHCO-NHCH-COOH y-Glu Cys G GSH is a kind of co-enzyme,playing an important role in oxidation-reduction system inside living cells. -2H 2GSH GSSG +2B Oxytocin or pitocin and vasopressin(ADH)Both molecules contain nine amino acids and produced by the clavage of polypeptide pr cursors with different specialized cells in After synthesis,they are ransported down the posterior pituitary gland,where they are stored HjN"-Cys-Tyr-IIe-Gln-Asn-Cys-Pro-Leu-Gly-C-ONH2 Oxytocin HjN'-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-C-ONHz vasopressin Adrenocorticotrophin or adrenocorticotropic hormone (ACTH)Produced in brain and hypothala a peptide consists of 39 amino ateaeFupcIonstosimulaiethesynthessandGectionofadrenalcoiealhormoe Opioid peptides Enkephalins:a pentapeptides containing 5 amino acids Met-enkephalin H-Tyr-Gly-Gly-Phe-Met-OH Leu-enkephalin:H-Tyr-Gly-Gly-Phe-Leu-OH.They function as to relieve pain and produce pleasant sensation,a role similar to opiate drugs such as morphine
19 component amino acids. These reactions are familiar to us from the above sections and from the study of organic chemistry and need not be repeated here. 2.3.3 Some natural peptides Glutathione (GSH) GSH is the most abundant anti-oxidant in the body. It is a tripeptide, made up of cysteine, glutamic acid, and glycine. It is the cystine part that donates the sulphur containing sulphydral group that is important in chelation. Glutathione is special in that it is fat soluble and water soluble, therefore it has anti-oxidant activity throughout the entire body. SH │ COOH CH2 │ │ H2NCHCH2CH2CO NHCHCO NHCH ━ ━ 2COOH γ-Glu Cys Gly GSH is a kind of co-enzyme, playing an important role in oxidation-reduction system inside living cells. -2H 2GSH GSSG +2H Oxytocin or pitocin and vasopressin(ADH) Both molecules contain nine amino acids and produced by the cleavage of polypeptide precursors with different specialized cells in hypothalamus . After synthesis, they are transported down nerve tracts into the posterior pituitary gland, where they are stored. S S │ │ H3N+ -Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-C-ONH2 Oxytocin S S │ │ H3N+ -Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-C-ONH2 vasopressin Adrenocorticotrophin or adrenocorticotropic hormone (ACTH) Produced in adenohypophysis 、 brain 、 and hypothalamus. Structure: a peptide consists of 39 amino acids.Functions:to stimulate the synthesis and ejection of adrenal cortical hormone. Opioid peptides Enkephalins: a pentapeptides containing 5 amino acids Met-enkephalin: H-Tyr-Gly-Gly-Phe-Met-OH Leu-enkephalin: H- Tyr-Gly-Gly-Phe-Leu-OH. They function as to relieve pain and produce pleasant sensation, a role similar to opiate drugs such as morphine
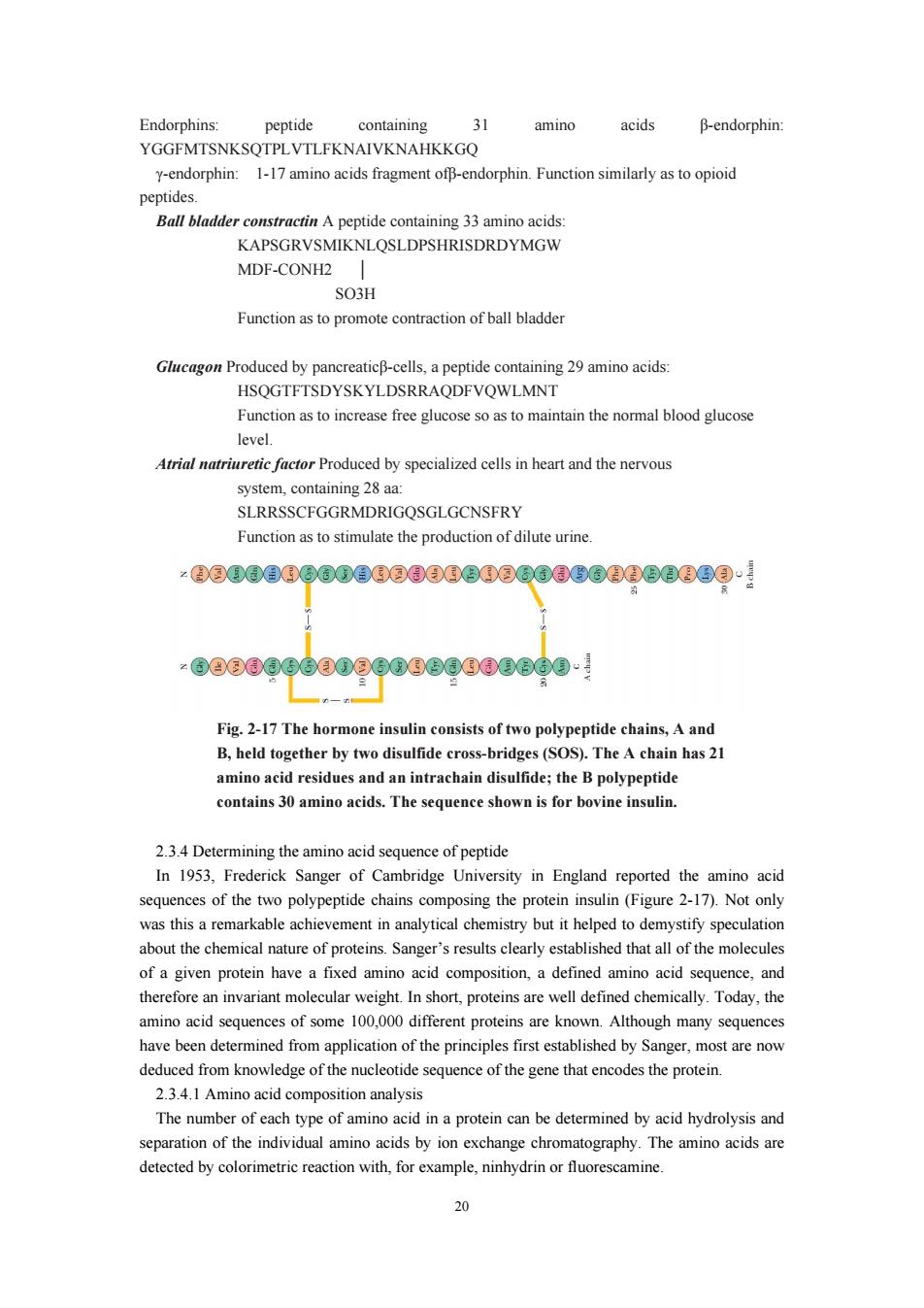
Endornhins pentide containing 31 amino acids B-endorphin: YGGEMTSNKSOTPLVTLEKNAIVKNAHKKGO -endorphin 1-17amino acids fragment-endorphin.Function smlarly as to peptide Ball bladder constractin A peptide containing 33 amino acids KAPSGRVSMIKNLOSLDPSHRISDRDYMGW MDF-CONH2 SO3H Function as to promote conraction of ball bladde Glucagon Produced by pancreaticB-cells,a peptide containing 29 amino acids: HSQGTFTSDYSKYLDSRRAQDFVQWLMNT Function as to increase free glucose so as to maintain the normal blood glucose Atrial natriuretic factor Produced by specialized cells in heart and the nervous system,containing 28 aa: SurrSSCEGGRMDRIGOSGLGONSERY Function as to stimulate the production of dilute urine. :6080g0600g60g80880600· Fig.2-17 The hormone insulin consists of two polypeptide chains,Aan B,held together by two disulfide cross-bridges (SOS).The A chain has 21 amino acid residues and an intrachain disulfide;the B polypeptide contains 30 amino acids.The sequence shown is for bovine insulin. 2.3.4 Determining the amino acid sequence of peptide In 1953.Frederick Sanger of Cambridge University in England reported the amino acid sequences of the two polypeptide chains composing the protein insulin (Figure 2-17).Not only was this a remarkable achievement in analytical chemistry but it helped to demystify speculation about the chemical nature of proteins.Sanger's results carly established that all of the molecules of a given protein havea fixed amino acid composition,a defined amin o acid sequence,and therefore an invariant molecular weight.In short,proteins are well defined chemically.Today,the amino acid sequences of some 100,000 different proteins are known.Although many sequences have been determined from application of the principles first established by Sanger,most are now deduced from knowledge of the nucleotide sequence of the gene that encodes the protein. 24.1Amino acid composition analysi The number of each type of amino acid in a protein can be determined by acid hydrolysis and separation of the individual amino acids by ion exchange chromatography.The amino acids are detected by colorimetric reaction with,for example,ninhydrin or fluorescamine. 20
20 Endorphins: peptide containing 31 amino acids β-endorphin: YGGFMTSNKSQTPLVTLFKNAIVKNAHKKGQ γ-endorphin: 1-17 amino acids fragment ofβ-endorphin. Function similarly as to opioid peptides. Ball bladder constractin A peptide containing 33 amino acids: KAPSGRVSMIKNLQSLDPSHRISDRDYMGW MDF-CONH2 │ SO3H Function as to promote contraction of ball bladder Glucagon Produced by pancreaticβ-cells, a peptide containing 29 amino acids: HSQGTFTSDYSKYLDSRRAQDFVQWLMNT Function as to increase free glucose so as to maintain the normal blood glucose level. Atrial natriuretic factor Produced by specialized cells in heart and the nervous system, containing 28 aa: SLRRSSCFGGRMDRIGQSGLGCNSFRY Function as to stimulate the production of dilute urine. 2.3.4 Determining the amino acid sequence of peptide In 1953, Frederick Sanger of Cambridge University in England reported the amino acid sequences of the two polypeptide chains composing the protein insulin (Figure 2-17). Not only was this a remarkable achievement in analytical chemistry but it helped to demystify speculation about the chemical nature of proteins. Sanger’s results clearly established that all of the molecules of a given protein have a fixed amino acid composition, a defined amino acid sequence, and therefore an invariant molecular weight. In short, proteins are well defined chemically. Today, the amino acid sequences of some 100,000 different proteins are known. Although many sequences have been determined from application of the principles first established by Sanger, most are now deduced from knowledge of the nucleotide sequence of the gene that encodes the protein. 2.3.4.1 Amino acid composition analysis The number of each type of amino acid in a protein can be determined by acid hydrolysis and separation of the individual amino acids by ion exchange chromatography. The amino acids are detected by colorimetric reaction with, for example, ninhydrin or fluorescamine. Fig. 2-17 The hormone insulin consists of two polypeptide chains, A and B, held together by two disulfide cross-bridges (SOS). The A chain has 21 amino acid residues and an intrachain disulfide; the B polypeptide contains 30 amino acids. The sequence shown is for bovine insulin

2.34.2 Edman degradation The N-terminal amino cid of a be determined by reacting the protein with dansy enzen prior to acid hydrolysi The amino acid sequence of a protein can be determined by Edman degradation which sequentially removes one residue at a time from the N-terminus 2.3.4.3 Sequencing strategy fragments using ither che als (e cyanogen bromide)or enzymes (e.chymo Trypsin).The resulting smaller fragments are sequenced by Edman degradation.The complet sequence is assembled by analyzing overlapping fragments generated by cleaving the polypeptide with different reagents. 2 3 44 New progress in protein sequencing Nowadays as little as picomole amounts of proteins can be seque nced following their following their transfer to nitrocellulose. Presently,recombinant DNA technology has enabled the sequences of even very large proteins to be determined by first sequencing the stretch of DNA encoding protein and then using the decipher the protein equence. Currently,protein sequencing and DNA sequencing are techniques that are used together to determine the complete sequence of protein 2.3.5 Primary structure of protein 2 3 5 1 Primary structure The primary level of structure in a protein is the linear sequence of amino acids as joined together by gene encoding the protein.Also included under primary structure is the location of any othe covalent bonds.These are primary disulfide bonds between cysteine residues that are adjacent in space but not in the linear amino acid sequence.These covalent cross-links between separate polypeptide chains or between different parts of the same chain are formed by the oxidation of the SH g residues that are space.Disulfide bonds present in ular proteins.Some proteins,suchas collagen,have covalent cross-links formed between the side-chains of Lys residues. 2.3.5.2 The relationship with function Proteins have unique amino acid sequences.and it is this uniqueness of sequence that ultimately gives each protein its own particular personality.Because the number of possible amino acid will,by chanc have similar amino acidq Consequently,sequence similarities betweer proteins imply evolutionary relatedness Homologous proteins from different organisms have homologous amino acid sequences. Proteins sharing a significant degree of sequence similarity are said to be homologous.Proteins that perform the same function in differe organisms are also referred to as homologous.For example,the oxygen transport protein,hemoglobin,serves a similar role and has a simila structure in all vertebrates.The study of the amino acid sequences of homologous proteins from different organisms provides very strong evidence for their evolutionary origin within a common ancestor.Homologous proteins characteristically have polypeptide chains that are nearly identical
21 2.3.4.2 Edman degradation The N-terminal amino acid of a protein can be determined by reacting the protein with dansyl chloride or fluorodinitrobenzene prior to acid hydrolysis. The amino acid sequence of a protein can be determined by Edman degradation which sequentially removes one residue at a time from the N-terminus. 2.3.4.3 Sequencing strategy In order to sequence an entire protein, the polypeptide chain has to be broken down into smaller fragments using either chemicals (e.g. cyanogen bromide) or enzymes (e.g. chymotrypsin and Trypsin ). The resulting smaller fragments are sequenced by Edman degradation. The complete sequence is assembled by analyzing overlapping fragments generated by cleaving the polypeptide with different reagents. 2.3.4.4 New progress in protein sequencing Nowadays, as little as picomole amounts of proteins can be sequenced following their separation by SDS-PAGE either using the polyacrylamide gel containing the protein directly, or following their transfer to nitrocellulose. Presently, recombinant DNA technology has enabled the sequences of even very large proteins to be determined by first sequencing the stretch of DNA encoding protein and then using the genetic code to decipher the protein sequence. Currently, protein sequencing and DNA sequencing are techniques that are used together to determine the complete sequence of protein. 2.3.5 Primary structure of protein 2.3.5.1 Primary structure The primary level of structure in a protein is the linear sequence of amino acids as joined together by peptide bonds. This sequence is determined by the sequence of nucleotide bases in the gene encoding the protein. Also included under primary structure is the location of any other covalent bonds. These are primary disulfide bonds between cysteine residues that are adjacent in space but not in the linear amino acid sequence. These covalent cross-links between separate polypeptide chains or between different parts of the same chain are formed by the oxidation of the SH groups on cysteine residues that are justaposed in space. Disulfide bonds are often present in extracellular proteins, but are rarely found in intracellular proteins. Some proteins, such as collagen, have covalent cross-links formed between the side-chains of Lys residues. 2.3.5.2 The relationship with function Proteins have unique amino acid sequences, and it is this uniqueness of sequence that ultimately gives each protein its own particular personality. Because the number of possible amino acid sequences in a protein is astronomically large, the probability that two proteins will, by chance, have similar amino acid sequences is negligible. Consequently, sequence similarities between proteins imply evolutionary relatedness. Homologous proteins from different organisms have homologous amino acid sequences. Proteins sharing a significant degree of sequence similarity are said to be homologous. Proteins that perform the same function in different organisms are also referred to as homologous. For example, the oxygen transport protein, hemoglobin, serves a similar role and has a similar structure in all vertebrates. The study of the amino acid sequences of homologous proteins from different organisms provides very strong evidence for their evolutionary origin within a common ancestor. Homologous proteins characteristically have polypeptide chains that are nearly identical
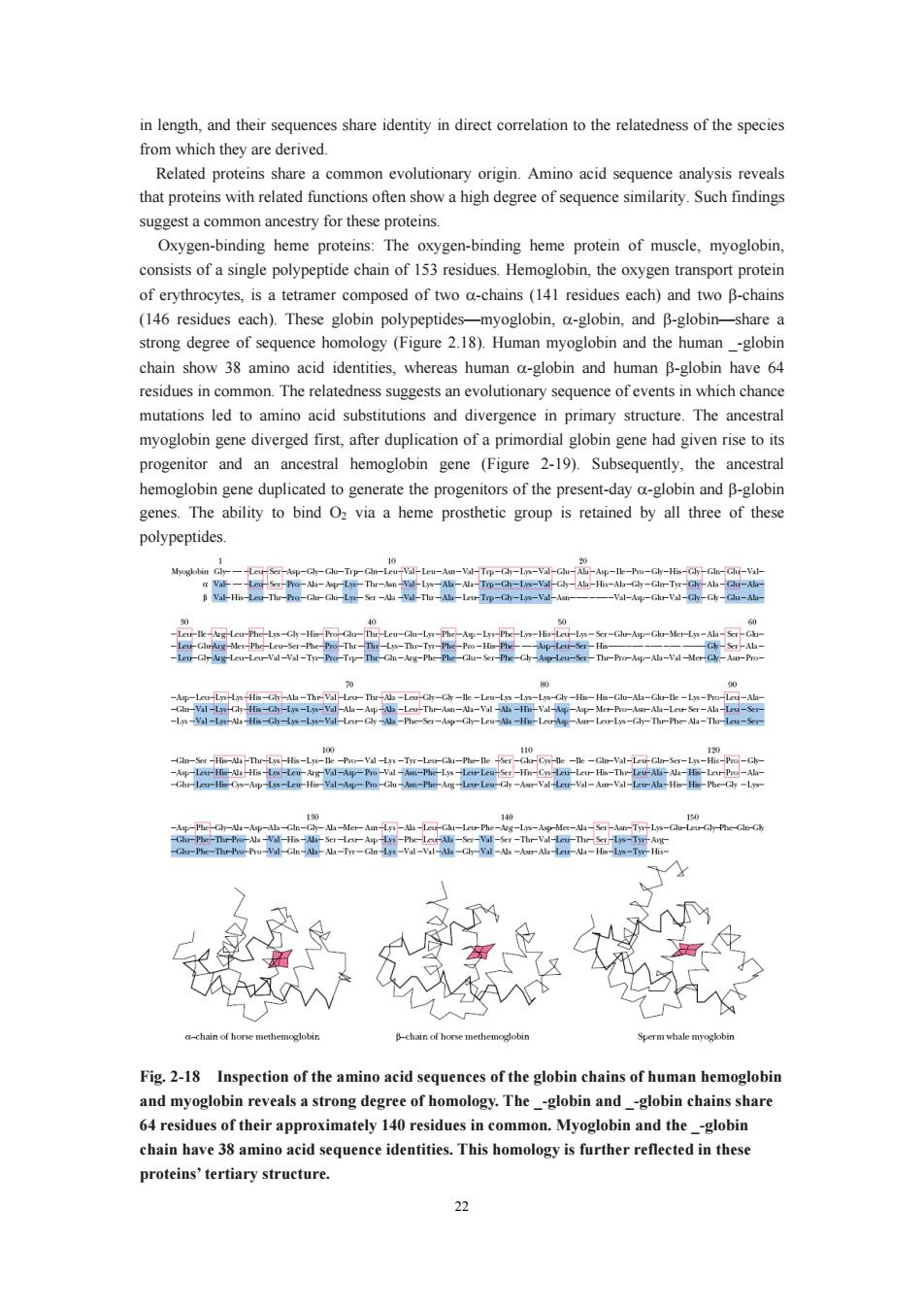
in length.and their sequences share identity in direct correlation to the relatedness of the species from which they are derived. common evolutionary reveals that proteins with related functions ofen show a high degree of sequence similarity.Such findings suggest a common ancestry for these proteins. Oxygen-binding heme proteins:The oxygen-binding heme protein of muscle,myoglobin. consists of a single polypeptide chain of 153 residues.Hemoglobin,the oxygen transport protein is a te ramer composed of two a-chains (141 residues each)and two B-chains (146 residues each).These globin polypeptide -myoglobin,a-globin,and B-globin hare a strong degree of sequence homology (Figure 2.18).Human myoglobin and the human-globin chain show 38 amino acid identities,whereas human a-globin and human B-globin have 64 residues in common.The relatedness suggests an evolutionary sequence of events in which chance mutations led toamino acid substitutions and divergence in primary structure.The ancestral gene diverged first after dup progenitor and an ancestral hemoglobin gene (Figure 2-19).Subsequently,the ancestral hemoglobin gene duplicated to generate the progenitors of the present-day a-globin and B-globin genes.The ability to bind O2 via a heme prosthetic group is retained by all three of these polypeptides. -nehe-Vin 2 6 Fig.2-18 Inspection of the amino acid sequences of the globin chains of human hemoglobin and myoglobin reveals a strong degree of homology.The -globin and -globin chains share 64 residues of their approximately 140 residues in common.Myoglobin and the-globin chain have 38 amino acids proteins'tertiary structure 22
22 in length, and their sequences share identity in direct correlation to the relatedness of the species from which they are derived. Related proteins share a common evolutionary origin. Amino acid sequence analysis reveals that proteins with related functions often show a high degree of sequence similarity. Such findings suggest a common ancestry for these proteins. Oxygen-binding heme proteins: The oxygen-binding heme protein of muscle, myoglobin, consists of a single polypeptide chain of 153 residues. Hemoglobin, the oxygen transport protein of erythrocytes, is a tetramer composed of two α-chains (141 residues each) and two β-chains (146 residues each). These globin polypeptides—myoglobin, α-globin, and β-globin—share a strong degree of sequence homology (Figure 2.18). Human myoglobin and the human _-globin chain show 38 amino acid identities, whereas human α-globin and human β-globin have 64 residues in common. The relatedness suggests an evolutionary sequence of events in which chance mutations led to amino acid substitutions and divergence in primary structure. The ancestral myoglobin gene diverged first, after duplication of a primordial globin gene had given rise to its progenitor and an ancestral hemoglobin gene (Figure 2-19). Subsequently, the ancestral hemoglobin gene duplicated to generate the progenitors of the present-day α-globin and β-globin genes. The ability to bind O2 via a heme prosthetic group is retained by all three of these polypeptides. Fig. 2-18 Inspection of the amino acid sequences of the globin chains of human hemoglobin and myoglobin reveals a strong degree of homology. The _-globin and _-globin chains share 64 residues of their approximately 140 residues in common. Myoglobin and the _-globin chain have 38 amino acid sequence identities. This homology is further reflected in these proteins’ tertiary structure
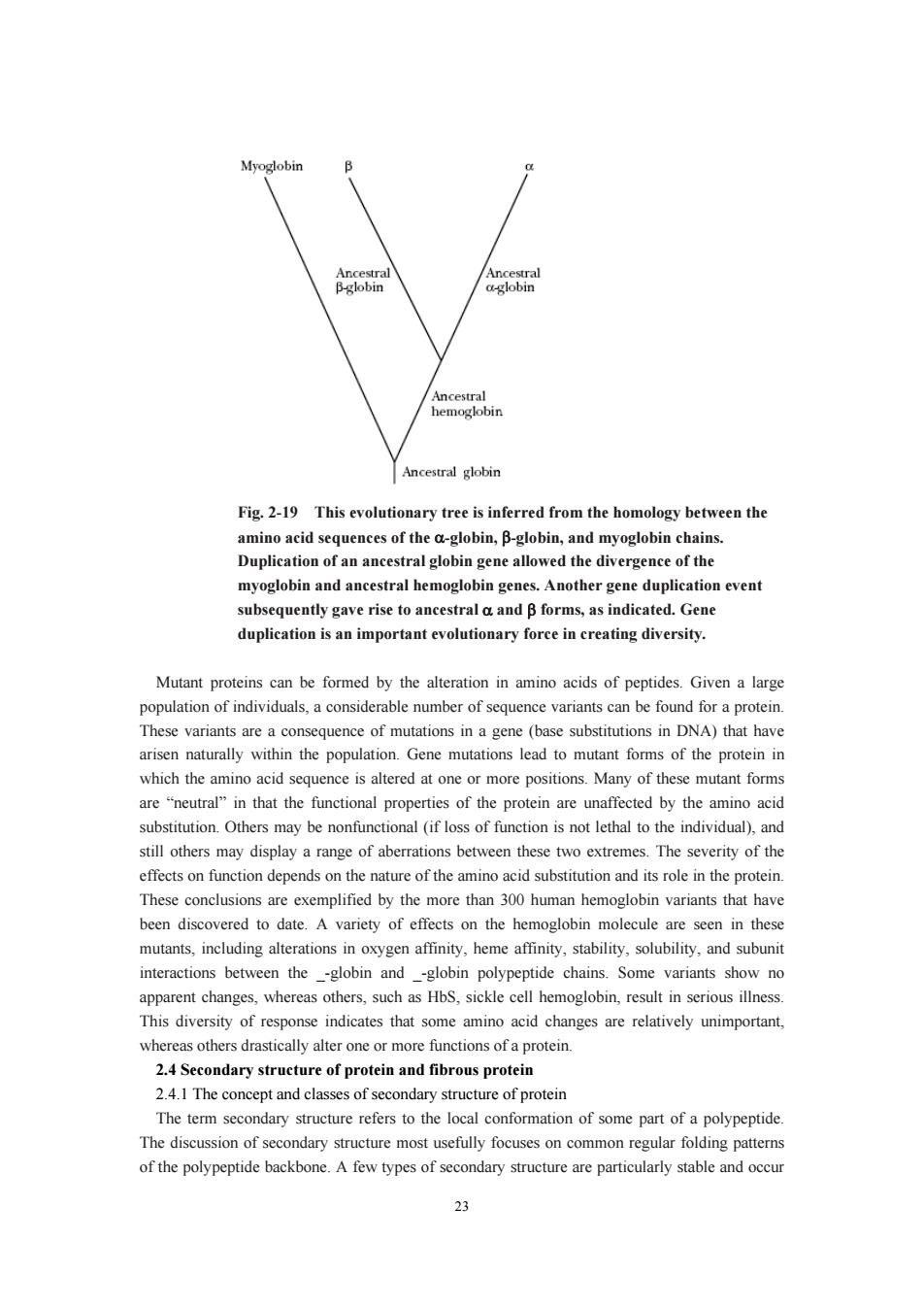
Myoglobin Bsobin hmobobin Fig.2-19 This evolutionary tree is inferred from the homology between the amino acid Duplicati myoglobin and ancestral hemoglobin genes.Another gene duplication event subsequently gave rise to ancestral a and B forms,as indicated.Gene duplication is an important evolutionary force in creating diversity. Mutant proteins can be formed by the alteration in amino acids of peptides.Given a larg population of individuals,a considerable number of sequence variants can be found for a proteir These variants are a consequence of mutations in a gene (base substitutions in DNA)that have arisen naturally within the population.Gene mutations lead to mutant forms of the protein in which the amino acid sequence is altered at one or more positions.Many of these mutant forms are"that the functional properties of the protein are unaffected by the amino acid may be onfun onal (if oss of function is o ethal to the individual)an still others may display a range of aberrations between these two extremes.The severity of the effects on function depends on the nature of the amino acid substitution and its role in the protein These conclusions are exemplified by the more than 300 human hemoglobin variants that have erdtodate.A variety of eftso the hemoglobin mo oxygen,heme and subun interactions between the-globin and-globin polypeptide chains.Some variants show no apparent changes.whereas others.such as HbS.sickle cell hemoglobin.result in serious illness This diversity of response indicates that some amino acid changes are relatively unimportant, whereas others drastically alter one or more functions of a protein. 2.4 Secondary str ture of protein and fibrous protein 2.4.1The concept and classes of econdary structure of protein The term secondary structure refers to the local conformation of some part of a polypeptide. The discussion of secondary structure most usefully focuses on common regular folding patterns of the polypeptide backbone.A few types of secondary structure are particularly stable and occur 23
23 Fig. 2-19 This evolutionary tree is inferred from the homology between the amino acid sequences of the α-globin, β-globin, and myoglobin chains. Duplication of an ancestral globin gene allowed the divergence of the myoglobin and ancestral hemoglobin genes. Another gene duplication event subsequently gave rise to ancestral α and β forms, as indicated. Gene duplication is an important evolutionary force in creating diversity. Mutant proteins can be formed by the alteration in amino acids of peptides. Given a large population of individuals, a considerable number of sequence variants can be found for a protein. These variants are a consequence of mutations in a gene (base substitutions in DNA) that have arisen naturally within the population. Gene mutations lead to mutant forms of the protein in which the amino acid sequence is altered at one or more positions. Many of these mutant forms are “neutral” in that the functional properties of the protein are unaffected by the amino acid substitution. Others may be nonfunctional (if loss of function is not lethal to the individual), and still others may display a range of aberrations between these two extremes. The severity of the effects on function depends on the nature of the amino acid substitution and its role in the protein. These conclusions are exemplified by the more than 300 human hemoglobin variants that have been discovered to date. A variety of effects on the hemoglobin molecule are seen in these mutants, including alterations in oxygen affinity, heme affinity, stability, solubility, and subunit interactions between the _-globin and _-globin polypeptide chains. Some variants show no apparent changes, whereas others, such as HbS, sickle cell hemoglobin, result in serious illness. This diversity of response indicates that some amino acid changes are relatively unimportant, whereas others drastically alter one or more functions of a protein. 2.4 Secondary structure of protein and fibrous protein 2.4.1 The concept and classes of secondary structure of protein The term secondary structure refers to the local conformation of some part of a polypeptide. The discussion of secondary structure most usefully focuses on common regular folding patterns of the polypeptide backbone. A few types of secondary structure are particularly stable and occur