
内蒙古农业大学 英汉双语教学辅助教材 生物化学选读 冯福应周欢敏主编 内蒙古农业大学教务处 二O0八年三月
内蒙古农业大学 英汉双语教学辅助教材 生物化学选读 冯福应 周欢敏 主编 内蒙古农业大学教务处 二〇〇八年三月

1.Introduction of Biochemistry In biology we are introduced to the extraordinary diversity of living organisms,a diversity that be classified.If we limit our inspection of living things to the gross organismic level,it is easy to be overwhelmed by this complexity.Furthermore,we will not find explanations for why organisms are constructed the way the are at this level of inquiry.If we probe more deeply into organismic structure.we find that cells are composed of a limited number of subcellular structures find the ultimate explanations for organismic structure and behavior.We should revel in the discovery that while living organisms are very complicated,there is a common chemistry and common rules that explain how they work. The object of this text is to acquaint the student of biochemistry with the basic facts and We will begin with an overview of some features of biochemistry as a biological principle. 1.I Biochemistry Is Chemistry of Living Organisms 1.1.1 The Concept of Biochemistry Life phenomena are unique and special motion of matters,in biological terms they are variant century,we now understand life are composed of molecules such as protein,nucleic acid,lipid and etc,and just they work together is to produce rich and colorful metabolism and life.And so,what are these molecules included,how are they generated and disposed and how do they change into and interact between each other?To discover the answers of these questions is the study content of biochemistry. Therefore the biochemistry,aso canbe called biological chemistry,has been defined as the study of the chemical nature of live beings and the chemical rule in the process of life activities. The chemistry of live beings resembles the chemistry of organic reaction at the molecule degree. Though the molecules are lifeless,in appreciate complexity and number they compose the live beings biochemical phenomena with cell biology,physiology,genetics,microbiology and molecular biology.though biochemistry is a basic one. 1.12 Basic chemical properties of living organisms 1.1.2.1 All living organisms consist of variant of biomolecules The diversity of nisms dep asmall bacterium,exampe about five thousands different species of molecules,including three thousands species of protein and one thousand species of nucleic acid.The sizes of biomolecules vary from very small to huge,and they appear in simple type or polymer.All different molecules have their own unique and special function.Interestingly,all
1 1. Introduction of Biochemistry In biology we are introduced to the extraordinary diversity of living organisms, a diversity that is so great that taxonomists generally acknowledge that there are many more species than will ever be classified. If we limit our inspection of living things to the gross organismic level, it is easy to be overwhelmed by this complexity. Furthermore, we will not find explanations for why organisms are constructed the way the are at this level of inquiry. If we probe more deeply into organismic structure, we find that cells are composed of a limited number of subcellular structures, macromolecules and small molecules. We also find that the macromolecules, which make up the bulk of the solid matter of cells, are closely related in structure. It is at the molecular level that we find the ultimate explanations for organismic structure and behavior. We should revel in the discovery that while living organisms are very complicated, there is a common chemistry and common rules that explain how they work. The object of this text is to acquaint the student of biochemistry with the basic facts and principles of this subject. We will begin with an overview of some features of biochemistry as a biological principle. 1.1 Biochemistry Is Chemistry of Living Organisms 1.1.1 The Concept of Biochemistry Life phenomena are unique and special motion of matters; in biological terms they are variant processes of metabolism. Thanks to the investigation of thousand of biochemists over the past century, we now understand life are composed of molecules such as protein, nucleic acid, lipid and, etc, and just they work together is to produce rich and colorful metabolism and life. And so, what are these molecules included, how are they generated and disposed and how do they change into and interact between each other? To discover the answers of these questions is the study content of biochemistry. Therefore the biochemistry, also can be called biological chemistry, has been defined as the study of the chemical nature of live beings and the chemical rule in the process of life activities. The chemistry of live beings resembles the chemistry of organic reaction at the molecule degree. Though the molecules are lifeless, in appreciate complexity and number they compose the live beings. Biochemistry is a growing discipline closely linked to other fields. For this reason, relate the biochemical phenomena with cell biology, physiology, genetics, microbiology and molecular biology, though biochemistry is a basic one. 1.1.2 Basic chemical properties of living organisms 1.1.2.1 All living organisms consist of variant of biomolecules The diversity of organisms depends on molecules diversity and their structural complexity. Take a small bacterium, Escherichia coli, as example, it contains about five thousands different species of molecules, including three thousands species of protein and one thousand species of nucleic acid. The sizes of biomolecules vary from very small to huge, and they appear in simple type or polymer. All different molecules have their own unique and special function. Interestingly, all

organisms practice molecular economy,that they would not construct the complexity over their well-arranged molecu ystems Though large verities of biomolecules exist in organisms,they are well arranged rather than out-of-ordered,even a single-celled organism have well-organized structures.Moreover the biomolecules organization precisely control every biochemical reaction,whatever in a single-celled organisms in which hundreds of reaction undergo at the same time.or in multi-celled ismsown much complicated 1.12.3All living organisms need enzyme as catalyst for metabolic reactior Enzymes function as catalysts that change the rate of a reaction without being change themselves.Virtually all enzymes are proteins.though some catalvtically RNAs have been identified.Enzymes are highly specific and their activity can be regulated.As reaction catalyst,the specialty and efficiency of enzymes far excel those of inorganic catalysts 1.12All living organisms require energy Energy is required to meet bioactivities and the energy conveys are agreed with the Second Law of thermodynamics.All bio-energy is originated from the Sun and the transformation of light energy to chemical energy is carried out by plant and some bacteria with photosynthesis.In food-web,photosynthetic plant and bacteria utilize sun energy to synthesize carbohydrates and act as producer,while animal and other microorganisms eat the above nu s and they play as cons mers 1.1.2.5 All living organisms store their genetic information in genes Self reproducing drive the generation shifts and therefore it is most dominant profile of live beings.The genetic information required by self-reproduce is lied in gene composed of nucleic nces.DNAismain aterials of mjor live beings and t oducing ability As to the features of biochemistry above,biochemistry is divided into three main basic fields chemistry of biomolecule,metabolism(materials and energy)and molecular genetics. 12 The Content and Intention of Biochemistry Biochemistry studies substance composition,propert s.metabolism.molecule structure and function.With the aim the hasic la and studying methods in biology chemis and physics biochemistry study the composition and structure.physical-and-chemical properties structure and function of chemical substance in organisms,from angle of biology and chemistry. respectively. Biochemistry aims to make students known the relationships between/among protein.enzyme nucleic acid,carbohydrate,lipid,vitamin and hormone,gr n of enzyme in catabolism of thr Jor cellula structural substances protein,lipid and carbohydrate,and understood the relationship betweer structure and function of biomacromolecules and basic rule of metabolic regulation.Mainly at the degree of molecule,biochemistry probes the basic structure,space structure:biological properties and function of biomolecu and also at relatively macroscopic cellular and subcellular degree investigate the dist 1.3 The Simple History of Biochemistry As one of most ancient branch of life science,biochemistry was originated from physiology and organic chemistry.Modern biochemistry started at the early time of 18 century and became an
2 organisms practice molecular economy, that they would not construct the complexity over their requirements. 1.1.2.2 All living organisms are complicate and well-arranged molecular systems Though large verities of biomolecules exist in organisms, they are well arranged rather than out-of-ordered, even a single-celled organism have well-organized structures. Moreover the biomolecules organization precisely control every biochemical reaction, whatever in a single-celled organisms in which hundreds of reaction undergo at the same time, or in multi-celled organisms own much complicated organized structures and control systems. 1.1.2.3 All living organisms need enzyme as catalyst for metabolic reaction Enzymes function as catalysts that change the rate of a reaction without being change themselves. Virtually all enzymes are proteins, though some catalytically RNAs have been identified. Enzymes are highly specific and their activity can be regulated. As reaction catalyst, the specialty and efficiency of enzymes far excel those of inorganic catalysts. 1.1.2.4 All living organisms require energy Energy is required to meet bioactivities and the energy conveys are agreed with the Second Law of thermodynamics. All bio-energy is originated from the Sun and the transformation of light energy to chemical energy is carried out by plant and some bacteria with photosynthesis. In food-web, photosynthetic plant and bacteria utilize sun energy to synthesize carbohydrates and act as producer, while animal and other microorganisms eat the above nutrients and they play as consumers. 1.1.2.5 All living organisms store their genetic information in genes Self reproducing drive the generation shifts and therefore it is most dominant profile of live beings. The genetic information required by self-reproduce is lied in gene composed of nucleic sequences. DNA is main genetic materials of major live beings and its self-reproducing ability enables the live beings similar ability. As to the features of biochemistry above, biochemistry is divided into three main basic fields, chemistry of biomolecule, metabolism (materials and energy) and molecular genetics. 1.2 The Content and Intention of Biochemistry Biochemistry studies substance composition, properties, metabolism, molecule structure and function in organisms. With the aim of the basic law and studying methods in biology, chemistry and physics, biochemistry study the composition and structure, physical-and-chemical properties, structure and function of chemical substance in organisms, from angle of biology and chemistry, respectively. Biochemistry aims to make students known the relationships between/among protein, enzyme, nucleic acid, carbohydrate, lipid, vitamin and hormone, grasped the application of enzyme in laboratory and industry, mastered basic rules of anabolism and catabolism of three major cellular structural substances protein, lipid and carbohydrate, and understood the relationship between structure and function of biomacromolecules and basic rule of metabolic regulation. Mainly at the degree of molecule, biochemistry probes the basic structure, space structure; biological properties and function of biomolecule, and also at relatively macroscopic cellular and subcellular degree investigate the distribution and role of biomolecules. 1.3 The Simple History of Biochemistry As one of most ancient branch of life science, biochemistry was originated from physiology and organic chemistry. Modern biochemistry started at the early time of 18 century and became an
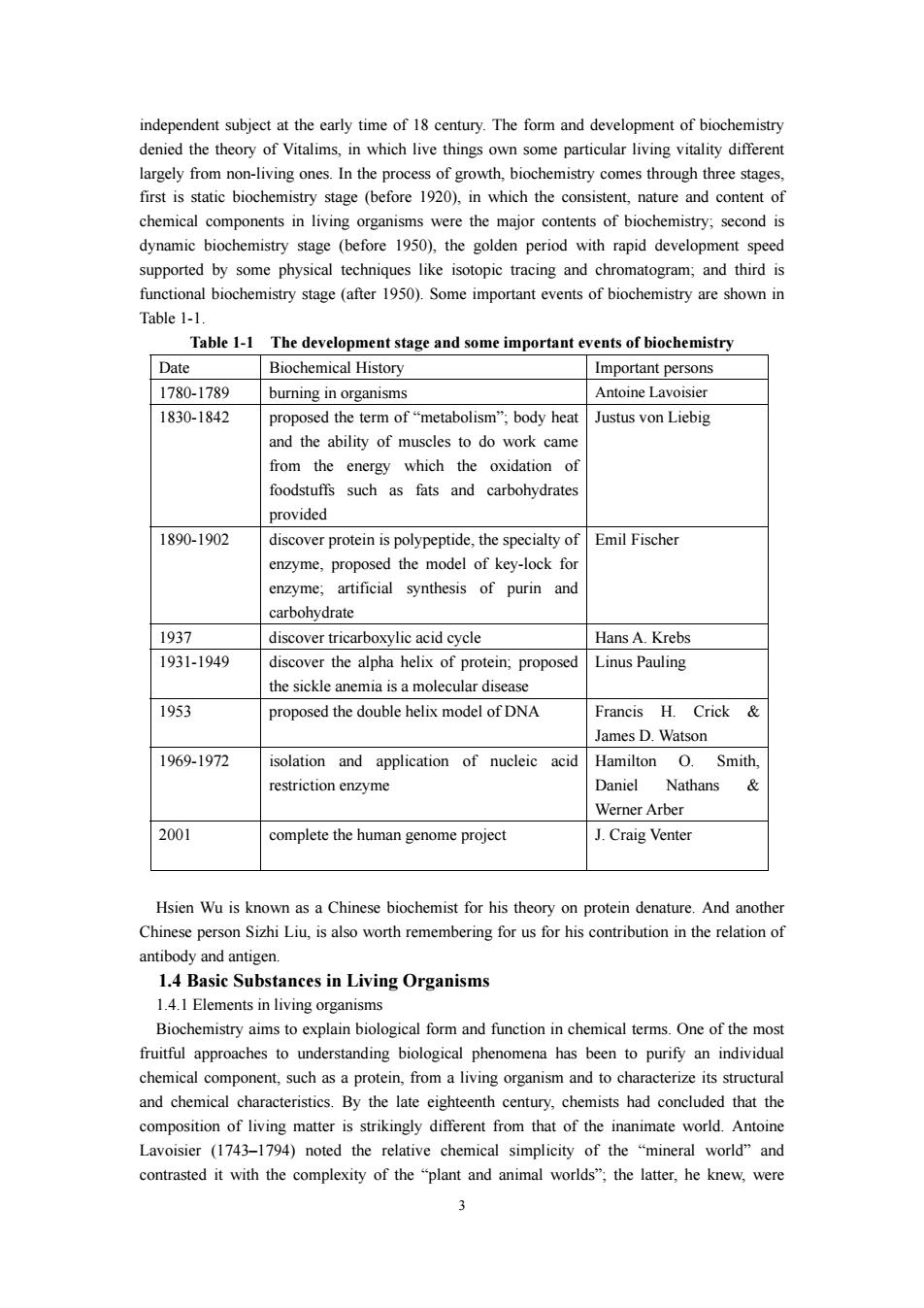
independent subiect at the early time of 18 century.The form and development of biochemistry denied the theory of Vitalims,in which live things ow largely f rom ving ones.In the process of growth, hrough three stage first is static biochemistry stage (before 1920),in which the consistent,nature and content of chemical components in living organisms were the major contents of biochemistry:second is dynamic biochemistry stage (before 1950).the golden period with rapid development speed supported by some physical techniques like isotopic tracing and chromatogram:and third is istry stage (after Some important events of bioche nistry are shown in Table 1-1 Table 1-1 The development stage and some important events of biochemistry Date Biochemical History Important persons 1780.1789 uring in organisms Antoine lavoisier 1830-1842 proposed the term ofmetabolism"body hea Justus von Liebig and the ability of muscles to do work cam from the energy which the oxidation of foodstuffs such as fats and carbohydrate provided 1890-1902 discover protein is polypeptide.the specialtyof Emil Fischer enzyme.proposed the model of key-lock fo enzyme:artificial synthesis of purin and carhohydrate 1037 Hans A.Krebs 1931-1949 discover the alpha helix of protein,proposed Linus Pauling the sickle anemia is a molecular disease 1953 proposed the double helix model of DNA Francis H.Crick& James d watson 1969-1972 isolation and application of nucleic acid Hamilton Smith, restriction enzyme Daniel Nathans Werner Arber 2001 complete the human genome project J.Craig Venter Hsien Wu is known as a Chinese biochemist for his theory on protein denature.And another Chinese person Sizhi Liu,is also worth remembering for us for his contribution in the relation of antibody and antigen 1.4 Basic Substances in Living Organisms 1.4.1 Elements in living organisms Biochemistry aims to explain biological form and function in chemical terms.One of the mos fruitful approaches to understanding biological phenomena has been to purify an individual chemical component.such as a protein.from a living organism and to characterize its structural and chemical characteristics by the late eighteenth century chemists had concluded that the matter is from that of the inanimate world.Antoine Lavoisier (1743-1794)noted the relative chemical simplicity of the "mineral world"and contrasted it with the complexity of the "plant and animal worlds";the latter,he knew,were 3
3 independent subject at the early time of 18 century. The form and development of biochemistry denied the theory of Vitalims, in which live things own some particular living vitality different largely from non-living ones. In the process of growth, biochemistry comes through three stages, first is static biochemistry stage (before 1920), in which the consistent, nature and content of chemical components in living organisms were the major contents of biochemistry; second is dynamic biochemistry stage (before 1950), the golden period with rapid development speed supported by some physical techniques like isotopic tracing and chromatogram; and third is functional biochemistry stage (after 1950). Some important events of biochemistry are shown in Table 1-1. Table 1-1 The development stage and some important events of biochemistry Date Biochemical History Important persons 1780-1789 burning in organisms Antoine Lavoisier 1830-1842 proposed the term of “metabolism”; body heat and the ability of muscles to do work came from the energy which the oxidation of foodstuffs such as fats and carbohydrates provided Justus von Liebig 1890-1902 discover protein is polypeptide, the specialty of enzyme, proposed the model of key-lock for enzyme; artificial synthesis of purin and carbohydrate Emil Fischer 1937 discover tricarboxylic acid cycle Hans A. Krebs 1931-1949 discover the alpha helix of protein; proposed the sickle anemia is a molecular disease Linus Pauling 1953 proposed the double helix model of DNA Francis H. Crick & James D. Watson 1969-1972 isolation and application of nucleic acid restriction enzyme Hamilton O. Smith, Daniel Nathans & Werner Arber 2001 complete the human genome project J. Craig Venter Hsien Wu is known as a Chinese biochemist for his theory on protein denature. And another Chinese person Sizhi Liu, is also worth remembering for us for his contribution in the relation of antibody and antigen. 1.4 Basic Substances in Living Organisms 1.4.1 Elements in living organisms Biochemistry aims to explain biological form and function in chemical terms. One of the most fruitful approaches to understanding biological phenomena has been to purify an individual chemical component, such as a protein, from a living organism and to characterize its structural and chemical characteristics. By the late eighteenth century, chemists had concluded that the composition of living matter is strikingly different from that of the inanimate world. Antoine Lavoisier (1743–1794) noted the relative chemical simplicity of the “mineral world” and contrasted it with the complexity of the “plant and animal worlds”; the latter, he knew, were
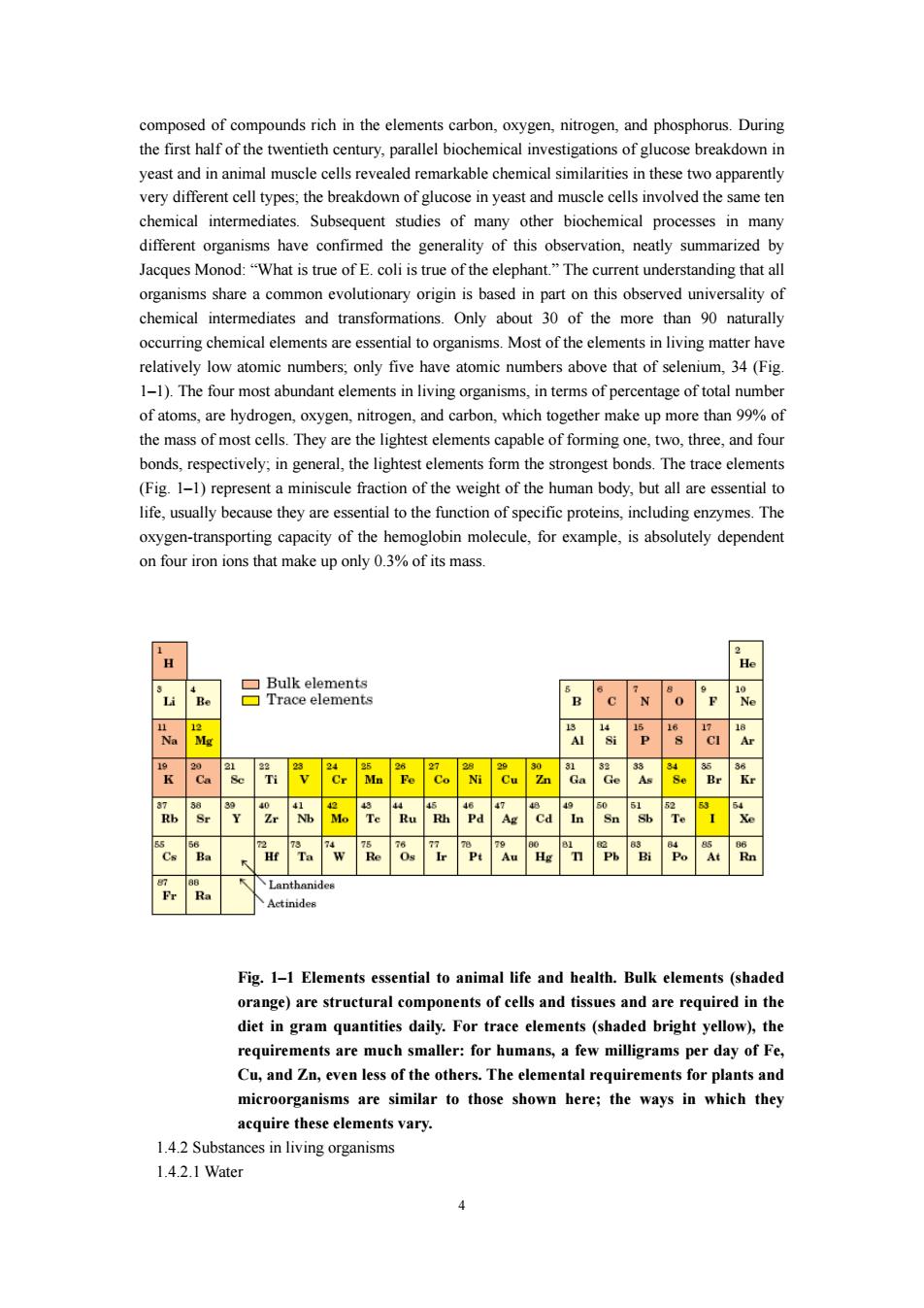
composed of compounds rich in the elements carbon.oxygen.nitrogen.and phosphorus.During yeast and n these two apparentl very different cell types,the breakdown of gucose in yeast and invoved the same te chemical intermediates.Subsequent studies of many other biochemical processes in many different organisms have confirmed the generality of this observation,neatly summarized by Jacques Monod:"What is true of E.coli is true of the elephant"The current understanding that all sality of chemical intermediates and transformations.Only abo occurring chemical elements are essential to organisms.Most of the elements in living matter have relatively low atomic numbers:only five have atomic numbers above that of selenium.34 (Fig 1-1).The four most abundant elements in living organisms.in terms of percentage of total number of atoms,are hydrogen,oxygen,nitrogen,and carbon,which together make up more than%of the mass ells They are capable of,and fou bonds,respectively;in general,the lightest elements form the strongest bonds.The trace element (Fig.1-1)represent a miniscule fraction of the weight of the human body,but all are essential to life,usually because they are essential to the function of specific proteins,including enzymes.The oxygen-transporting capacity of the hemoglobin molecule,for example,is absolutely dependent on four iron ions that make up only %fitsmas H 'u‘Re 已race ciement 'B"c 'x "o 'p Al ''s CI Ar k Ca be 'n v Cr Ma Pe Co M Cu in "da Ge be Br kr awzr.tu "it.Ya "Ae "Ca "'In "Sn.B飞 Ce Ba if Ta "W Re Os ir Pe Au ig T Ph Bi Po At in Fig.1-1 Elements essential to animal life and health.Bulk elements (shaded orange)are structural components of cells and tissues and are required in the diet in gram quantities daily.For trace elements (shaded bright yellow),the requirer nis are much er:for hu ans,a few milligrams per day of F Cu,and Zn,even less of the others.The elemental requirements for plants and microorganisms are similar to those shown here;the ways in which they acquire these elements vary. 1.4.2 Substances in living organisms 1.4.2.1Water
4 composed of compounds rich in the elements carbon, oxygen, nitrogen, and phosphorus. During the first half of the twentieth century, parallel biochemical investigations of glucose breakdown in yeast and in animal muscle cells revealed remarkable chemical similarities in these two apparently very different cell types; the breakdown of glucose in yeast and muscle cells involved the same ten chemical intermediates. Subsequent studies of many other biochemical processes in many different organisms have confirmed the generality of this observation, neatly summarized by Jacques Monod: “What is true of E. coli is true of the elephant.” The current understanding that all organisms share a common evolutionary origin is based in part on this observed universality of chemical intermediates and transformations. Only about 30 of the more than 90 naturally occurring chemical elements are essential to organisms. Most of the elements in living matter have relatively low atomic numbers; only five have atomic numbers above that of selenium, 34 (Fig. 1–1). The four most abundant elements in living organisms, in terms of percentage of total number of atoms, are hydrogen, oxygen, nitrogen, and carbon, which together make up more than 99% of the mass of most cells. They are the lightest elements capable of forming one, two, three, and four bonds, respectively; in general, the lightest elements form the strongest bonds. The trace elements (Fig. 1–1) represent a miniscule fraction of the weight of the human body, but all are essential to life, usually because they are essential to the function of specific proteins, including enzymes. The oxygen-transporting capacity of the hemoglobin molecule, for example, is absolutely dependent on four iron ions that make up only 0.3% of its mass. Fig. 1–1 Elements essential to animal life and health. Bulk elements (shaded orange) are structural components of cells and tissues and are required in the diet in gram quantities daily. For trace elements (shaded bright yellow), the requirements are much smaller: for humans, a few milligrams per day of Fe, Cu, and Zn, even less of the others. The elemental requirements for plants and microorganisms are similar to those shown here; the ways in which they acquire these elements vary. 1.4.2 Substances in living organisms 1.4.2.1 Water