
CH,-SH pA,-g he p for the Fig-1Effect of the chemical n pKa.The pKa values for the ionizable groups in glycine are lower than those for simple,methyl-substituted amino and carboxyl groups.These downward perturbations of pKa are due to intramolecular interactions.Similar effects can be caused by chemical groups that happen to be positioned nearby-for example,in the active site of an enzyme Another important piece of information derived from the titration curve of an amino acid is the relationship between its net electric charge and the pH of the solution.At pH 5.97.the point of inflection between the two stages in its titration curve,glycine is present predominantly as its dipolar form,fully ionized but with no net electric charge (Fig.2-10).The characteristic pH at which the net electric charge is zero is called the ic poi intor H.designated pl arithmetic mean of the two pKa values pl=。(pK1+pK)=。(2.34+9.60)=5.97 As is evident in Figure 3-10,glycine has a net negative charge at any pH above its pl and will thus move toward the(the anode )when placed ir electric field.At any pH cathode).The farther the pH of a glycine solution is from its isoelectric point,the greater the net electric charge of the population of glycine molecules.At pH 1.0.for example,glycine exists almost entirely as the form H3NOCH2OCOOH,with a net positive charge of 1.0.At pH 2.34. where there is an equal mixture of 'HN-CH2-COOH and'HN-CH-COO,the a erage or net positive charge is5.The sign and the magnitude of the net charge of any amino acid at any pH can be predicted in the same way 2 2.3.4 Chemical reactions of amino acids Carboxyl and Amino Group Reactions The -carboxyl and -amino groups of all amino acids exhibit similar chemical reactivity.The side chains.ho the study and analysis of isolated amino acids,it is the characteristic behavior of the side chain that governs the reactivity of amino acids incorporated into proteins.There are three reasons to consider these reactivities.Proteins can be chemically modified in very specifie ways by taking 14
14 Fig. 2–11 Effect of the chemical environment on pKa. The pKa values for the ionizable groups in glycine are lower than those for simple, methyl-substituted amino and carboxyl groups. These downward perturbations of pKa are due to intramolecular interactions. Similar effects can be caused by chemical groups that happen to be positioned nearby—for example, in the active site of an enzyme. Another important piece of information derived from the titration curve of an amino acid is the relationship between its net electric charge and the pH of the solution. At pH 5.97, the point of inflection between the two stages in its titration curve, glycine is present predominantly as its dipolar form, fully ionized but with no net electric charge (Fig. 2–10). The characteristic pH at which the net electric charge is zero is called the isoelectric point or isoelectric pH, designated pI. For glycine, which has no ionizable group in its side chain, the isoelectric point is simply the arithmetic mean of the two pKa values: 1 2 1 1 ( ) (2.34 9.60) 5.97 2 2 pI pK pK = += += As is evident in Figure 3–10, glycine has a net negative charge at any pH above its pI and will thus move toward the positive electrode (the anode) when placed in an electric field. At any pH below its pI, glycine has a net positive charge and will move toward the negative electrode (the cathode). The farther the pH of a glycine solution is from its isoelectric point, the greater the net electric charge of the population of glycine molecules. At pH 1.0, for example, glycine exists almost entirely as the form _H3NOCH2OCOOH, with a net positive charge of 1.0. At pH 2.34, where there is an equal mixture of + H3N-CH2-COOH and + H3N-CH2-COO- , the average or net positive charge is 0.5. The sign and the magnitude of the net charge of any amino acid at any pH can be predicted in the same way. 2.2.3.4 Chemical reactions of amino acids Carboxyl and Amino Group Reactions The _-carboxyl and _-amino groups of all amino acids exhibit similar chemical reactivity. The side chains, however, exhibit specific chemical reactivities, depending on the nature of the functional groups. Whereas all of these reactivities are important in the study and analysis of isolated amino acids, it is the characteristic behavior of the side chain that governs the reactivity of amino acids incorporated into proteins. There are three reasons to consider these reactivities. Proteins can be chemically modified in very specific ways by taking
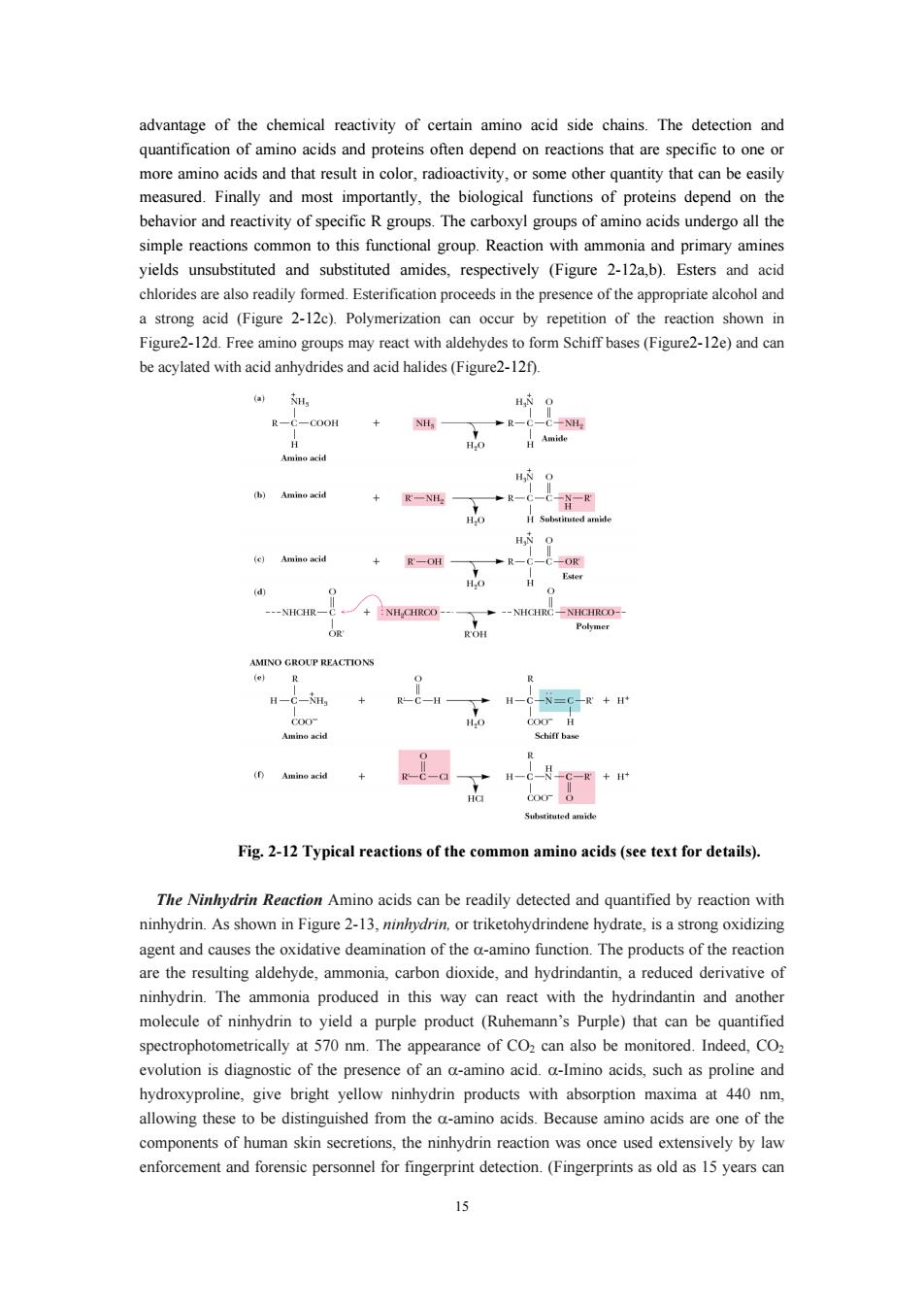
advantage of the chemical reactivity of certain amino acid side chains.The detection and more radioactivity,r some her quantity that can beeasi measured.Finally and most importantly,the biological functions of proteins depend on the behavior and reactivity of specific R groups.The carboxyl groups of amino acids undergo all the simple reactions common to this functional group.Reaction with ammonia and primary amines yields unsubstituted and substituted amides,respectively (Figure 2-12a,b).Esters and acid chlorides re also readily formed.Esterificatior n proc ds n the e pres e alcohol and a strong acid (Figure 2-12c).Polymerization can occur by repetition of the reaction shown in Figure2-12d.Free amino groups may react with aldehydes to form Schiff bases (Figure2-12e)and can be acylated with acid anhydrides and acid halides(Figure2-12). H. R-一C-C00H +NH -NICR-NICIROO- -NHGIRC-NHCRCO- H-C-H + H-N=g+ (Amine acid + 1-c-c-+ Fig.2-12 Typical reactions of the common amino acids(see text for details). The Ninlydrin Reaction Amino acids can be readily detected and quantified by reaction with ninhydrin.As shown in Figure 2-3.or triketohydrindene hydrate.is a strong oxidizing ctsof the eactio are the resulting aldehyde,ammonia,carbon dioxide,and hydrindantin,a reduced derivative of ninhydrin.The ammonia produced in this way can react with the hydrindantin and another molecule of ninhydrin to yield a purple product (Ruhemann's Purple)that can be quantified spectrophotometrically at 570 nm.The appearance of CO2 can also be monitored.Indeed,CO2 is diagnostic of the presence of an a-amino acid.a-Imino acids,such as proline and hydroxyproline,give bright yellow ninhydrin products with absorption maxima at 440nm allowing these to be distinguished from the a-amino acids.Because amino acids are one of the components of human skin secretions,the ninhydrin reaction was once used extensively by law enforcement and forensic personnel for fingerprint detection.(Fingerprints as old as 15 years can
15 advantage of the chemical reactivity of certain amino acid side chains. The detection and quantification of amino acids and proteins often depend on reactions that are specific to one or more amino acids and that result in color, radioactivity, or some other quantity that can be easily measured. Finally and most importantly, the biological functions of proteins depend on the behavior and reactivity of specific R groups. The carboxyl groups of amino acids undergo all the simple reactions common to this functional group. Reaction with ammonia and primary amines yields unsubstituted and substituted amides, respectively (Figure 2-12a,b). Esters and acid chlorides are also readily formed. Esterification proceeds in the presence of the appropriate alcohol and a strong acid (Figure 2-12c). Polymerization can occur by repetition of the reaction shown in Figure2-12d. Free amino groups may react with aldehydes to form Schiff bases (Figure2-12e) and can be acylated with acid anhydrides and acid halides (Figure2-12f). Fig. 2-12 Typical reactions of the common amino acids (see text for details). The Ninhydrin Reaction Amino acids can be readily detected and quantified by reaction with ninhydrin. As shown in Figure 2-13, ninhydrin, or triketohydrindene hydrate, is a strong oxidizing agent and causes the oxidative deamination of the α-amino function. The products of the reaction are the resulting aldehyde, ammonia, carbon dioxide, and hydrindantin, a reduced derivative of ninhydrin. The ammonia produced in this way can react with the hydrindantin and another molecule of ninhydrin to yield a purple product (Ruhemann’s Purple) that can be quantified spectrophotometrically at 570 nm. The appearance of CO2 can also be monitored. Indeed, CO2 evolution is diagnostic of the presence of an α-amino acid. α-Imino acids, such as proline and hydroxyproline, give bright yellow ninhydrin products with absorption maxima at 440 nm, allowing these to be distinguished from the α-amino acids. Because amino acids are one of the components of human skin secretions, the ninhydrin reaction was once used extensively by law enforcement and forensic personnel for fingerprint detection. (Fingerprints as old as 15 years can
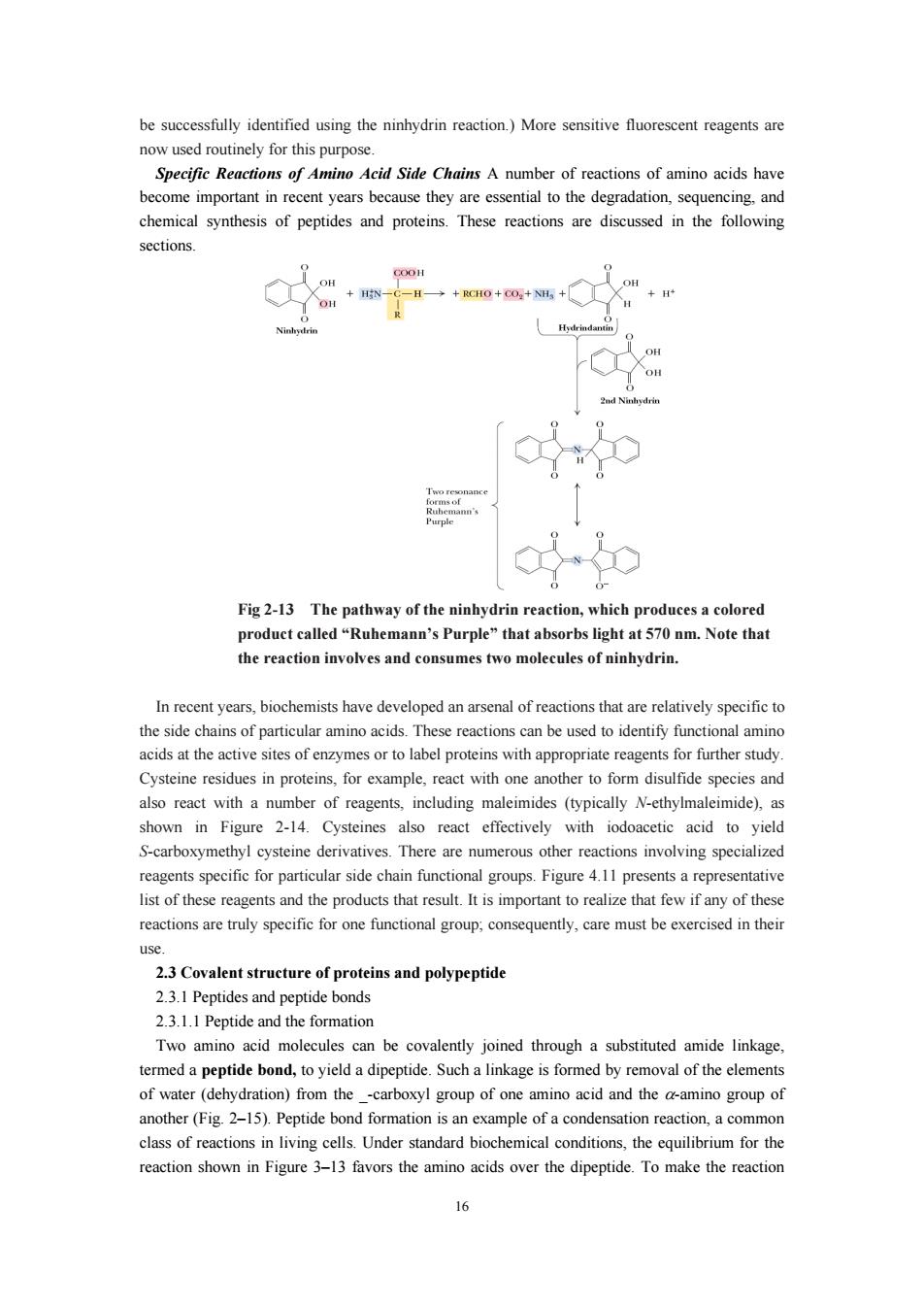
be successfully identified using the ninhydrin reaction)More sensitive fluorescent reagents are Specific Reactions f Amino Acid Side Chains A number of reactions of amino acids have become important in recent years because they are essential to the degradation,sequencing,and chemical synthesis of peptides and proteins.These reactions are discussed in the following sections. Fig2-13 The pathway of the ninhydrin reaction,which produces a colored product called"Ruhemann's Purple"that absorbs light at 570 nm.Note that the reaction involves and consumes two molecules of ninhydrin. nrecent years,biochemists have developed an arsenal of reactions that are relatively specifict the side chains of particular amino acids.These reactions can be used to identify functional amino acids at the active sites of enzymes or to label proteins with appropriate reagents for further study Cysteine residues in proteins,for example,react with one another to form disulfide species and also react with a number of reagents,including maleimides (typically N-ethylmaleimide),as shown in Figure 2-14.Cyste es also react effectively with iodoacetic acid to yield -carboxymethyl cysteine erivatives.There are umerous ther eactions specialized reagents specific for particular side chain functional groups.Figure 4.11 presents a representative list of these reagents and the products that result.It is important to realize that few if any of these reactions are truly specific for one functional group.consequently,care must be exercised in their 2.3Covalent structure of proteins and polypeptid 2.3.1 Peptides and peptide bonds 2.3.1.1 Peptide and the formation Two amino acid molecules can be covalently joined through a substituted amide linkage ota Kq pattuo s!aeyuil e yons 'apndadip e Ploi orp n)from the another(Fig 2-15).Peptide bond formation is an example of a condensation reaction,a commor class of reactions in living cells.Under standard biochemical conditions,the equilibrium for the reaction shown in Figure 3-13 favors the amino acids over the dipeptide.To make the reaction 16
16 be successfully identified using the ninhydrin reaction.) More sensitive fluorescent reagents are now used routinely for this purpose. Specific Reactions of Amino Acid Side Chains A number of reactions of amino acids have become important in recent years because they are essential to the degradation, sequencing, and chemical synthesis of peptides and proteins. These reactions are discussed in the following sections. Fig 2-13 The pathway of the ninhydrin reaction, which produces a colored product called “Ruhemann’s Purple” that absorbs light at 570 nm. Note that the reaction involves and consumes two molecules of ninhydrin. In recent years, biochemists have developed an arsenal of reactions that are relatively specific to the side chains of particular amino acids. These reactions can be used to identify functional amino acids at the active sites of enzymes or to label proteins with appropriate reagents for further study. Cysteine residues in proteins, for example, react with one another to form disulfide species and also react with a number of reagents, including maleimides (typically N-ethylmaleimide), as shown in Figure 2-14. Cysteines also react effectively with iodoacetic acid to yield S-carboxymethyl cysteine derivatives. There are numerous other reactions involving specialized reagents specific for particular side chain functional groups. Figure 4.11 presents a representative list of these reagents and the products that result. It is important to realize that few if any of these reactions are truly specific for one functional group; consequently, care must be exercised in their use. 2.3 Covalent structure of proteins and polypeptide 2.3.1 Peptides and peptide bonds 2.3.1.1 Peptide and the formation Two amino acid molecules can be covalently joined through a substituted amide linkage, termed a peptide bond, to yield a dipeptide. Such a linkage is formed by removal of the elements of water (dehydration) from the _-carboxyl group of one amino acid and the α-amino group of another (Fig. 2–15). Peptide bond formation is an example of a condensation reaction, a common class of reactions in living cells. Under standard biochemical conditions, the equilibrium for the reaction shown in Figure 3–13 favors the amino acids over the dipeptide. To make the reaction
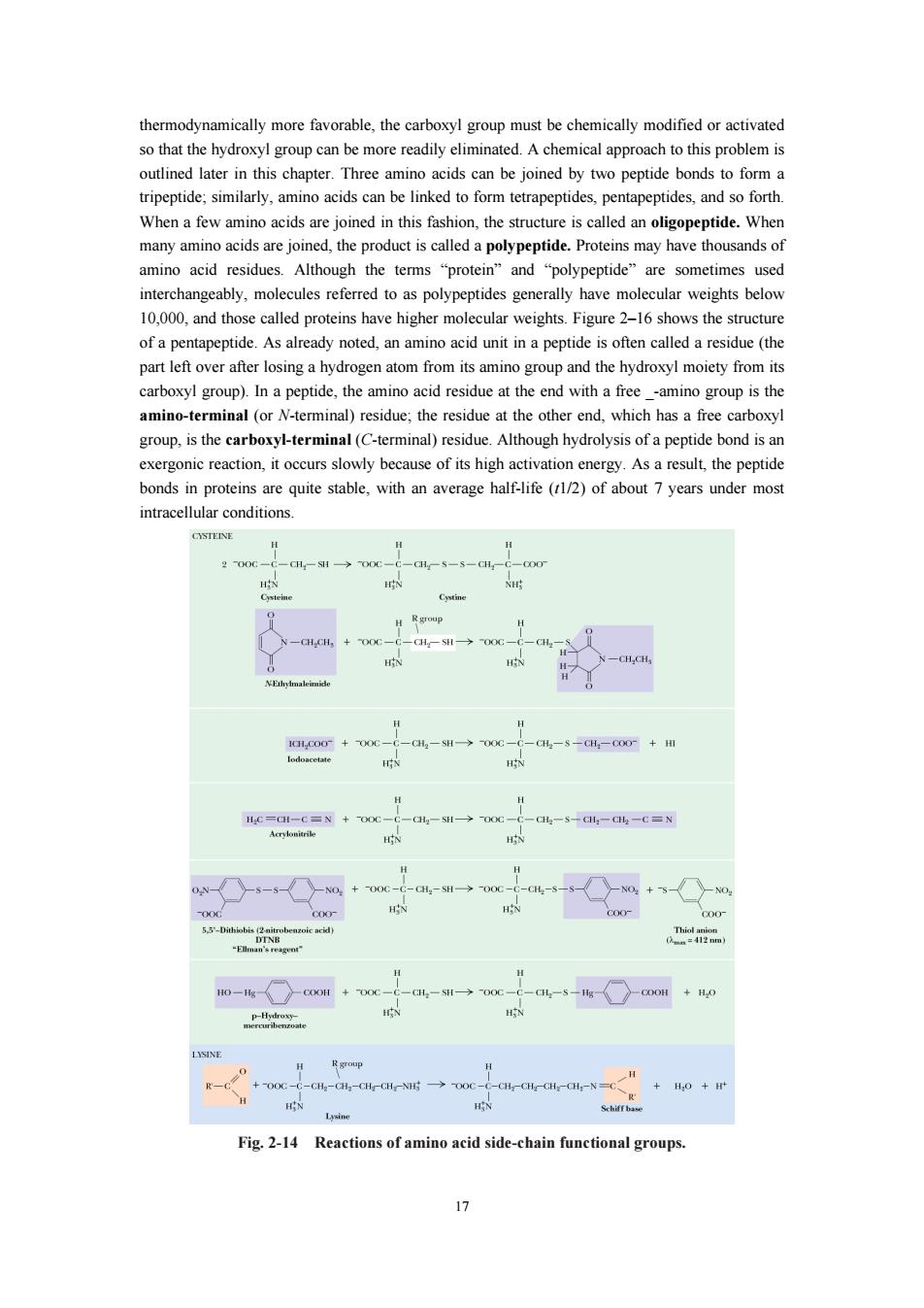
thermodynamically more favorable,the carboxyl group must be chemically modified or activated later in this chapter.Thee be oind onds to form a tripeptide:similarly,amino acids can be linked to form tetrapeptides,pentapeptides,and so forth When a few amino acids are joined in this fashion,the structure is called an oligopeptide.When many amino acids are joined,the product is called a polypeptide.Proteins may have thousands of amino acid residues.Although the terms "protein"and "polypeptide"are sometimes used cably,molecules oas polypeptides generally ha ave weights below 10.000.and those called proteins have higher molecular weights.Figure2-16 shows the structure of a pentapeptide.As already noted,an amino acid unit in a peptide is often called a residue (the part left over after losing a hydrogen atom from its amino group and the hydroxyl moiety from its carboxyl group)In a peptide,the amino acid residue at the end with a freeamno group is the amino-t minal (or N teminal)esidue,the residue at the which arboxyl group,is the caboxyl-terminal(C-terminal)residue.Although hydrolysis of a peptide bond isa exergonic reaction,it occurs slowly because of its high activation energy.As a result,the peptide bonds in proteins are quite stable,with an average half-life (1/2)of about 7 years under most intracellular conditions 1 N-GlCll,06- -a-→00 -dl X-o1g a+e-a4-期mc-o偶-5-0-m+m H 财aN+e 0○-s-s-Cn+c m-w○+ +0+ Fig.2-14 Reactions of amino acid side-chain functional groups
17 thermodynamically more favorable, the carboxyl group must be chemically modified or activated so that the hydroxyl group can be more readily eliminated. A chemical approach to this problem is outlined later in this chapter. Three amino acids can be joined by two peptide bonds to form a tripeptide; similarly, amino acids can be linked to form tetrapeptides, pentapeptides, and so forth. When a few amino acids are joined in this fashion, the structure is called an oligopeptide. When many amino acids are joined, the product is called a polypeptide. Proteins may have thousands of amino acid residues. Although the terms “protein” and “polypeptide” are sometimes used interchangeably, molecules referred to as polypeptides generally have molecular weights below 10,000, and those called proteins have higher molecular weights. Figure 2–16 shows the structure of a pentapeptide. As already noted, an amino acid unit in a peptide is often called a residue (the part left over after losing a hydrogen atom from its amino group and the hydroxyl moiety from its carboxyl group). In a peptide, the amino acid residue at the end with a free _-amino group is the amino-terminal (or N-terminal) residue; the residue at the other end, which has a free carboxyl group, is the carboxyl-terminal (C-terminal) residue. Although hydrolysis of a peptide bond is an exergonic reaction, it occurs slowly because of its high activation energy. As a result, the peptide bonds in proteins are quite stable, with an average half-life (t1/2) of about 7 years under most intracellular conditions. Fig. 2-14 Reactions of amino acid side-chain functional groups
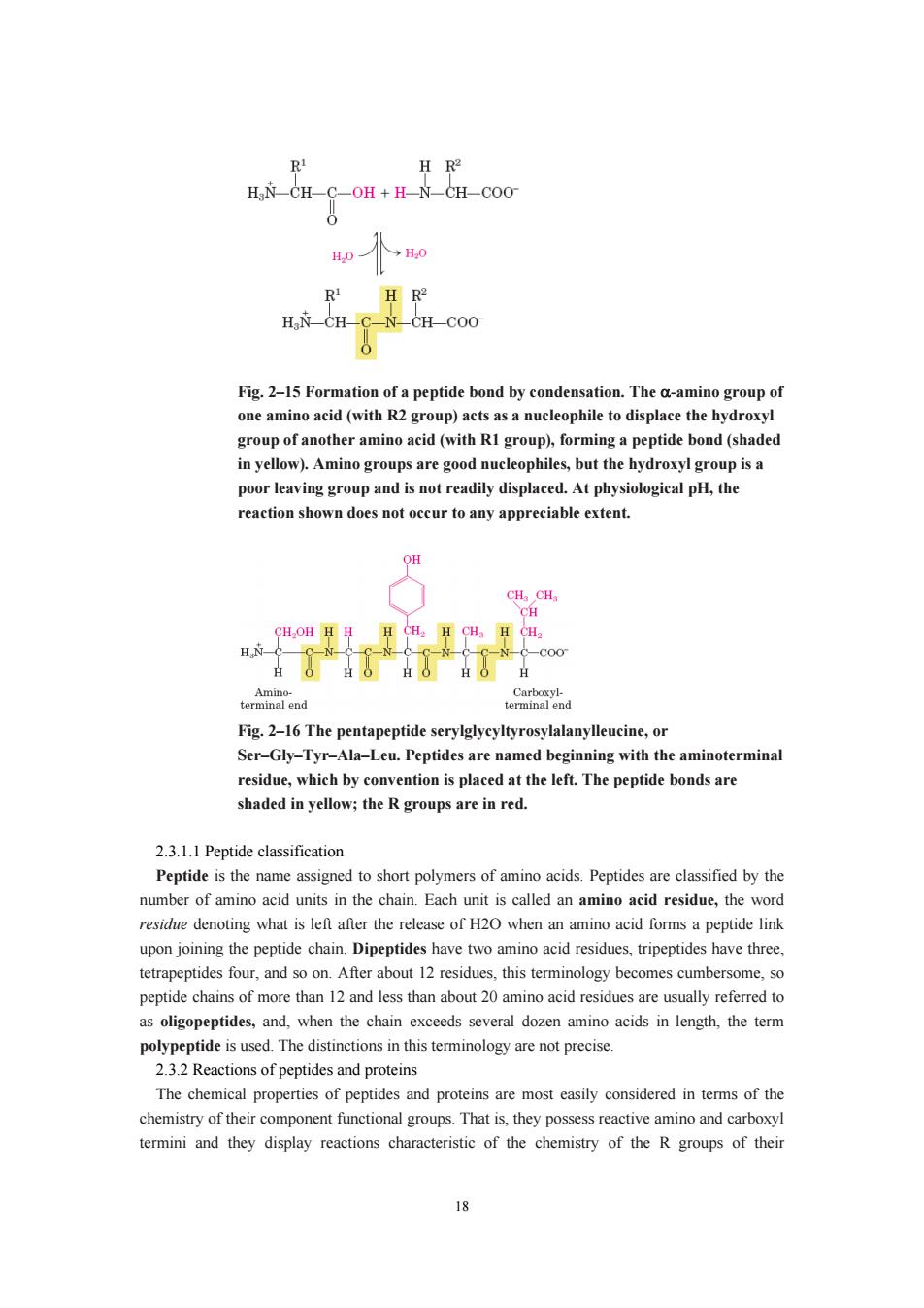
RI H R HN CH-C-OH+H N-CH-COO 0 →H0 H R H.N CH N-CH COO Fig.2-15 Formation of a peptide bond by condensation.The a-amino group of one amino acid(with R2 gr up)acts as nueleophile to displace the hyd group of a other amin acid (with RIgrou)forminga peptide bond (sh in yellow).Amino groups are good nucleophiles,but the hydroxyl group is a poor leaving group and is not readily displaced.At physiological pH,the reaction shown does not occur to any appreciable extent. OH Fig.2-16The pentapeptide serylglycyltyrosylalanylleucine,or Ser-Gly-Tyr-Ala-Leu.Peptides are named beginning with the aminoterminal residue,which by convention is placed at the left.The peptide bonds are shaded in yellow;the R groups are in red. 2.3.1.classification Peptide is the name assigned to short polymers of amino acids.Peptides are classified by the number of amino acid units in the chain.Each unit is called an amino acid residue,the word residue denoting what is left after the release of H20 when an amino acid forms a peptide link upon joining the peptide chain.Dipeptides havetwo aminoacid residues,tripeptides ha ave three. tetrapeptides four,about,this terminoloy becomes cumberm peptide chains of more than 12 and less than about 20 amino acid residues are usually referred to as oligopeptides,and,when the chain exceeds several dozen amino acids in length,the term polypeptide is used.The distinctions in this terminology are not precise. 23.Reactions of peptides and proteins The hemical properties of peptides and proteins re most easily onsidered in ters of th chemistry of their component functional groups.That is,they possess reactive amino and carboxyl termini and they display reactions characteristic of the chemistry of the R groups of their 18
18 Fig. 2–15 Formation of a peptide bond by condensation. The α-amino group of one amino acid (with R2 group) acts as a nucleophile to displace the hydroxyl group of another amino acid (with R1 group), forming a peptide bond (shaded in yellow). Amino groups are good nucleophiles, but the hydroxyl group is a poor leaving group and is not readily displaced. At physiological pH, the reaction shown does not occur to any appreciable extent. Fig. 2–16 The pentapeptide serylglycyltyrosylalanylleucine, or Ser–Gly–Tyr–Ala–Leu. Peptides are named beginning with the aminoterminal residue, which by convention is placed at the left. The peptide bonds are shaded in yellow; the R groups are in red. 2.3.1.1 Peptide classification Peptide is the name assigned to short polymers of amino acids. Peptides are classified by the number of amino acid units in the chain. Each unit is called an amino acid residue, the word residue denoting what is left after the release of H2O when an amino acid forms a peptide link upon joining the peptide chain. Dipeptides have two amino acid residues, tripeptides have three, tetrapeptides four, and so on. After about 12 residues, this terminology becomes cumbersome, so peptide chains of more than 12 and less than about 20 amino acid residues are usually referred to as oligopeptides, and, when the chain exceeds several dozen amino acids in length, the term polypeptide is used. The distinctions in this terminology are not precise. 2.3.2 Reactions of peptides and proteins The chemical properties of peptides and proteins are most easily considered in terms of the chemistry of their component functional groups. That is, they possess reactive amino and carboxyl termini and they display reactions characteristic of the chemistry of the R groups of their