
widely in proteins.The most prominent are the a helix and B conformations described below Using fundaental chemicalpinpdepratios Pa years before the firs In considering secondary structure,it is useful to classify proteins into two major groups fibrous proteins,having polypeptide chains arranged in long strands or sheets,and globular proteins,with polypeptide chains folded into a spherical or globular shape.Fibrous proteins play mportant structural oes in the anatomy and physiology of vertebrates,providing extema protection,support,shape.and form.They may constitute one-half or more of the total bod protein in larger animals.Most enzymes and peptide hormones are globular proteins.Globula proteins tend to be structurally complex.often containing several types of secondary structure fibrous proteins usually consist largely of a single type of secondary structure.Because of this structural simplicity,certain fibrous proteins played a key role in the development of the modern structure and vide clea examples of the relationshi etween structure and function.they are considered in some detail after the general discussionof secondary structure. 2.4.2 The folding of polypeptide and amide plane Apolypeptide chain can fold into a regularly repeating structure.In 1951,Linus Pauling and Robert Corey proposed two periodie structures alled thehelix and thepleated sheet Subsequently,other structures such as the tum and loop were identified.Although not periodic,these common turn or loop structures are well defined and contribute with helices and sheets to form the final protein structure. The planarity of the peptide bond means that there are only two degrees of freedom per residue for DpP网pmo andals about the bond linkingthe nitrogen of the peptide bond and the adjac a-carbon.As shown in Figure 2-20,each a-carbon is the joining point for two planes defined by peptide bonds.The angle about the CN bond is denoted by the Greek letter(phi)and that about the CCis denoted by(psi).For either of these bond angles,a value of corresponds to an hhe ehe)lne and oiuation the main chain question (Figure -1).Ina case,the entire path of the peptide backbone in a protein is known if theand rotation angles are all specified.Some values of and are not allowed due to steric interference between nonbonded atoms. Fig.2-20 The amide or peptide bond planes are joined by the tetrahedral bonds of the -carbon.The rotation parameters are and The conformation sho and180.Note that positive values of and P correspond to clockwise rotation as viewed from CaStarting from,a rotation of 180 in the clockwise direction (+180)is equivalent to a rotation of 180 in the direction(-180)
24 widely in proteins. The most prominent are the α helix and β conformations described below. Using fundamental chemical principles and a few experimental observations, Pauling and Corey predicted the existence of these secondary structures in 1951, several years before the first complete protein structure was elucidated. In considering secondary structure, it is useful to classify proteins into two major groups: fibrous proteins, having polypeptide chains arranged in long strands or sheets, and globular proteins, with polypeptide chains folded into a spherical or globular shape. Fibrous proteins play important structural roles in the anatomy and physiology of vertebrates, providing external protection, support, shape, and form. They may constitute one-half or more of the total body protein in larger animals. Most enzymes and peptide hormones are globular proteins. Globular proteins tend to be structurally complex, often containing several types of secondary structure; fibrous proteins usually consist largely of a single type of secondary structure. Because of this structural simplicity, certain fibrous proteins played a key role in the development of the modern understanding of protein structure and provide particularly clear examples of the relationship between structure and function; they are considered in some detail after the general discussion of secondary structure. 2.4.2 The folding of polypeptide and amide plane A polypeptide chain can fold into a regularly repeating structure. In 1951, Linus Pauling and Robert Corey proposed two periodic structures called the α helix and the β pleated sheet. Subsequently, other structures such as the β turn and Ω loop were identified. Although not periodic, these common turn or loop structures are well defined and contribute with helices and sheets to form the final protein structure. The planarity of the peptide bond means that there are only two degrees of freedom per residue for the peptide chain. Rotation is allowed about the bond linking the α-carbon and the carbon of the peptide bond and also about the bond linking the nitrogen of the peptide bond and the adjacent α-carbon. As shown in Figure 2-20, each α-carbon is the joining point for two planes defined by peptide bonds. The angle about the Cα—N bond is denoted by the Greek letter Ψ (phi) and that about the Cα—Co is denoted by φ (psi). For either of these bond angles, a value of 0° corresponds to an orientation with the amide plane bisecting the H—Cα—R (sidechain) plane and a cis configuration of the main chain around the rotating bond in question (Figure 2-21). In any case, the entire path of the peptide backbone in a protein is known if the φ and rotation angles are all specified. Some values of φ and Ψ are not allowed due to steric interference between nonbonded atoms. Fig. 2-20 The amide or peptide bond planes are joined by the tetrahedral bonds of the _-carbon. The rotation parameters are φ and Ψ. The conformation shown corresponds to φ = 180° and Ψ = 180°. Note that positive values of φ and Ψ correspond to clockwise rotation as viewed from Cα. Starting from 0°, a rotation of 180° in the clockwise direction (+180°) is equivalent to a rotation of 180° in the counterclockwise direction (-180°)

鉴章泛 Fig.2-21 Many of the possible conformations about an -carbon between two peptide planes are forbidden beeause of sterie crowding.Several noteworthy examples are shown here. 2.4.3 The a-helix The discussion of hydrogen bonding in Section 2.3.1 pointed out that the carbonyl oxygen and amide hydrogen of the peptide bond could participate in H bonds either with water molecules in the solvent or with other H-bonding groups in the peptide chain.In nearly all proteins.the arbonyl oxygens and the portions of the peptide chain.Structures resulting from these interactions constitute secondary structure for proteins.When a number of hydrogen bonds form between portions of the peptide chain in this manner,two basic types of structures can result:pleated shees Fig.2-20 Fig.2-22 A hydrogen bond between the amide proton and carbonyl oxygen of adjacent peptide groups. Evidence for helical structures in proteins was first obtained in the 1930s in studies of fibrous amino acid residues.(A single turn of the -helix involves 13 atoms from the O to the H of the H bond For this reason,the a-helix is sometimes referred to as the 3.6helix.)This is in fact the feature that most confused crystallographers before the Pauling and Corey ohelix model.Crystallographers were 25
25 Fig. 2-21 Many of the possible conformations about an _-carbon between two peptide planes are forbidden because of steric crowding. Several noteworthy examples are shown here. 2.4.3 The α-helix The discussion of hydrogen bonding in Section 2.3.1 pointed out that the carbonyl oxygen and amide hydrogen of the peptide bond could participate in H bonds either with water molecules in the solvent or with other H-bonding groups in the peptide chain. In nearly all proteins, the carbonyl oxygens and the amide protons of many peptide bonds participate in H bonds that link one peptide group to another, as shown in Figure 2-22. These structures tend to form in cooperative fashion and involve substantial portions of the peptide chain. Structures resulting from these interactions constitute secondary structure for proteins. When a number of hydrogen bonds form between portions of the peptide chain in this manner, two basic types of structures can result: α-helices and β-pleated sheets. Evidence for helical structures in proteins was first obtained in the 1930s in studies of fibrous proteins. Since that time later, the α-helix has proved to be a fundamentally important peptide structure. Several representations of the α-helix are shown in Figure 2-23. One turn of the helix represents 3.6 amino acid residues. (A single turn of the _-helix involves 13 atoms from the O to the H of the H bond. For this reason, the α-helix is sometimes referred to as the 3.613 helix.) This is in fact the feature that most confused crystallographers before the Pauling and Corey α-helix model. Crystallographers were Fig. 2-20 Fig. 2-22 A hydrogen bond between the amide proton and carbonyl oxygen of adjacent peptide groups
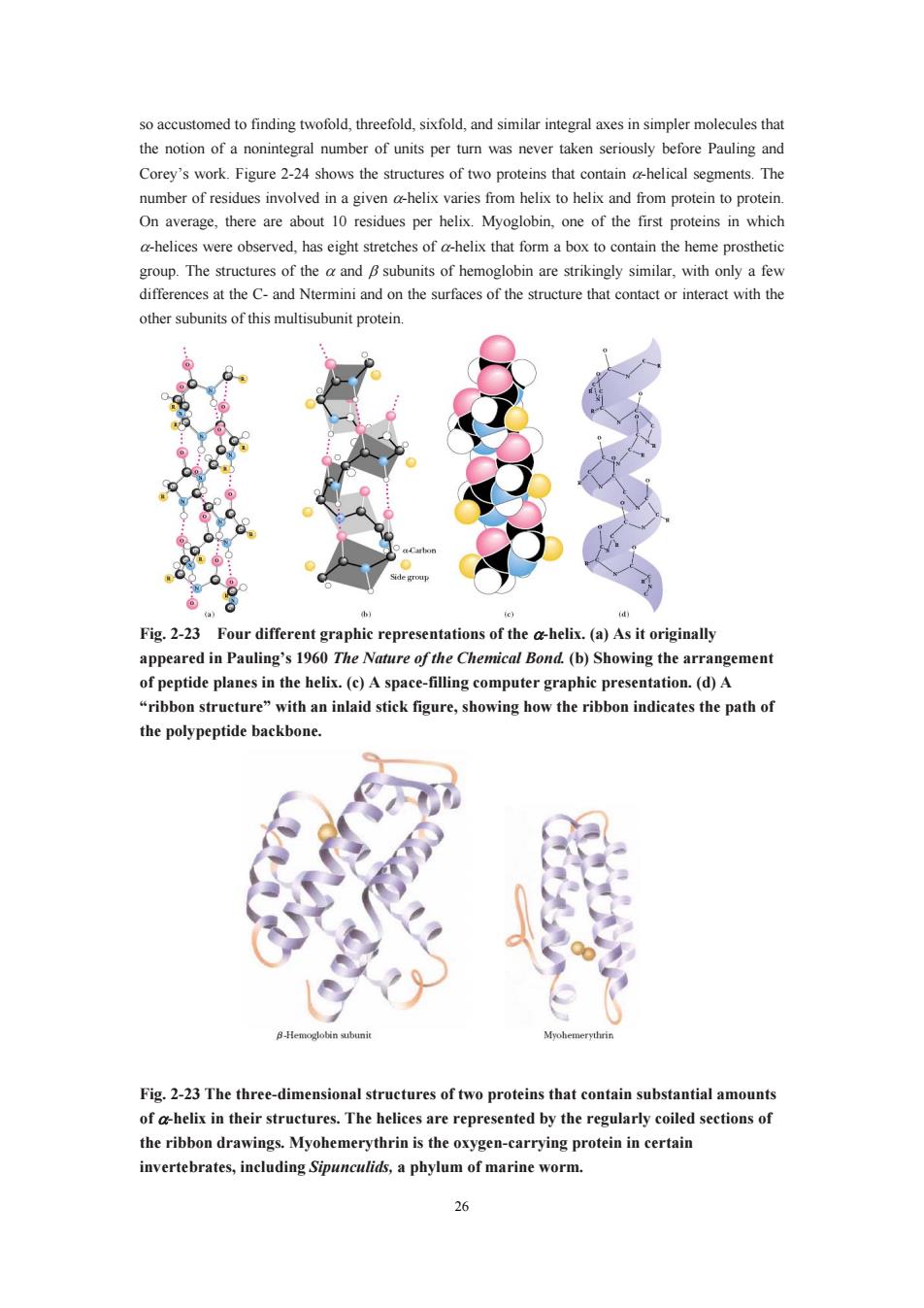
so accustomed to finding twofold,threefold,sixfold,and similar integral axes in simpler molecules that the notion of a nonintegral number of units per turn was never taken seriously before Pauling and Corey's work.Figure 2-24 shows the structures of two proteins that sements.The number of residues involved in a given a-helix varies from helix to helix and from protein to proteir On average,there are about 10 residues per helix.Myoglobin,one of the first proteins in which a-helices were observed,has eight stretches of a-helix that form a box to contain the heme prosthetic group.The structures of theand subunits of hemoglobin are strikingly similar,with only a few and on the surfaces of the structure that contact or interact with the ther subunits of this multisubunit proteir Fig.2-23 Four different graphic representations of the ahelix.(a)As it originally appeared in Pauling's 1960 The Nature of the Chemical Bond.(b)Showing the arrangement plesin th be spac-mutaipret ribbon structure ith an inlaid igure,sho ribbon indicates the path o the polypeptide backbone 8-Hemc Fig.2-23 The three-dimensional structures of two proteins that contain substantial amount of a-helix in their structures.The helices are represented by the regularly coiled sections of the ribbon drawings.Myohemerythrin is the oxygen-carrying protein in certain invertebrates,including Sipunculids,a phylum of marine worm. 26
26 so accustomed to finding twofold, threefold, sixfold, and similar integral axes in simpler molecules that the notion of a nonintegral number of units per turn was never taken seriously before Pauling and Corey’s work. Figure 2-24 shows the structures of two proteins that contain α-helical segments. The number of residues involved in a given α-helix varies from helix to helix and from protein to protein. On average, there are about 10 residues per helix. Myoglobin, one of the first proteins in which α-helices were observed, has eight stretches of α-helix that form a box to contain the heme prosthetic group. The structures of the α and β subunits of hemoglobin are strikingly similar, with only a few differences at the C- and Ntermini and on the surfaces of the structure that contact or interact with the other subunits of this multisubunit protein. Fig. 2-23 Four different graphic representations of the α-helix. (a) As it originally appeared in Pauling’s 1960 The Nature of the Chemical Bond. (b) Showing the arrangement of peptide planes in the helix. (c) A space-filling computer graphic presentation. (d) A “ribbon structure” with an inlaid stick figure, showing how the ribbon indicates the path of the polypeptide backbone. Fig. 2-23 The three-dimensional structures of two proteins that contain substantial amounts of α-helix in their structures. The helices are represented by the regularly coiled sections of the ribbon drawings. Myohemerythrin is the oxygen-carrying protein in certain invertebrates, including Sipunculids, a phylum of marine worm
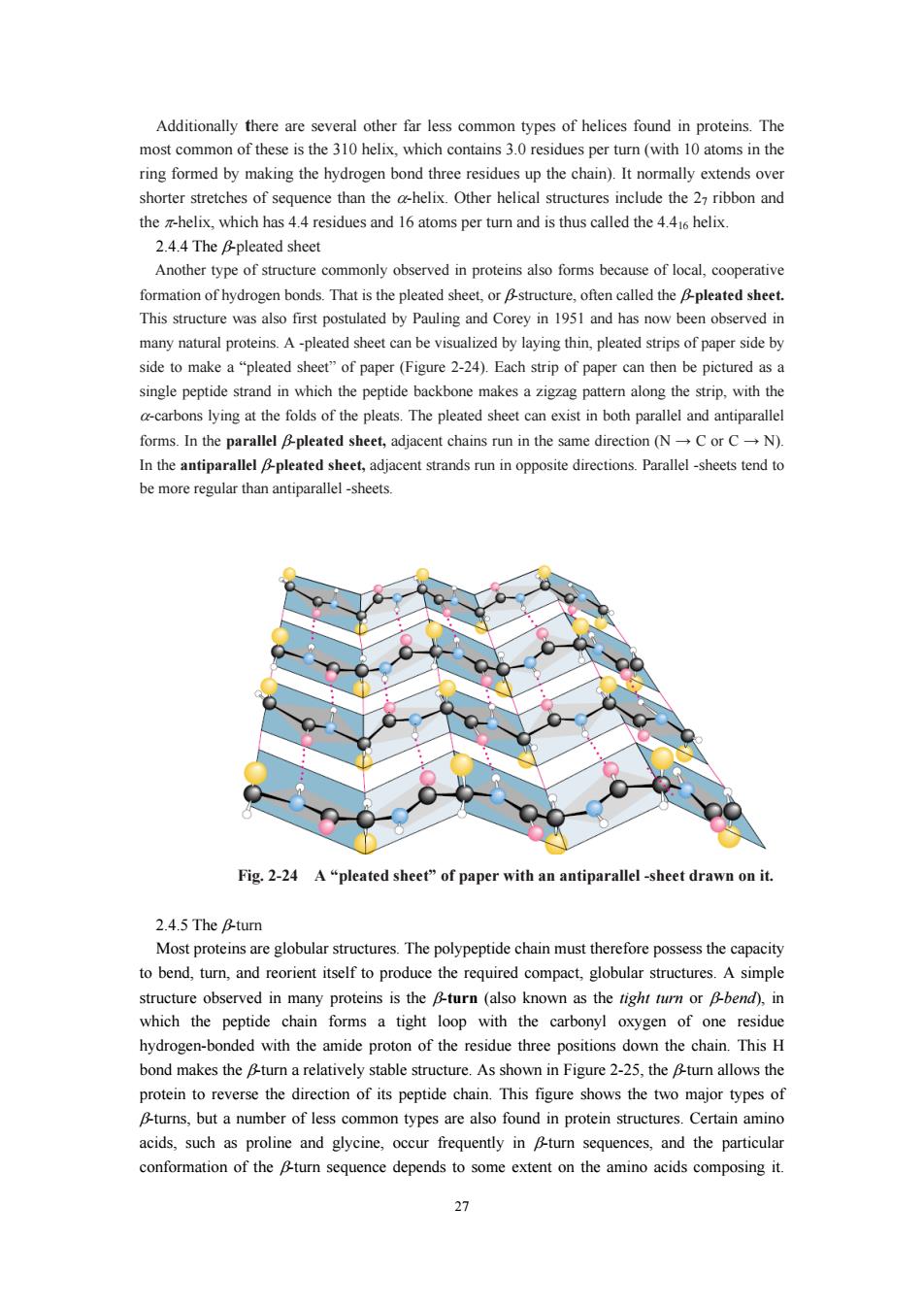
shorter stretches of sequence than the a-helix.Other helical structures include the 27 ribbon and the helix,which has 4.4 residues and 16 atoms per turn and is thus called the 4.4helix. 24.4 The &pleated sheet Another type of structure commonly observed in proteins also forms because of local,cooperative ormation bonds That is the pleated. This structure was also first postulated by Pauling and Corey in 1951 and has now been observed ir many natural proteins.A-pleated sheet can be visualized by laying thin,pleated strips of paper side by side to make a "pleated sheet"of paper (Figure 2-24).Each strip of paper can then be pictured as a single peptide strand in which the peptide backbone makes a zigzag pattern along the strip.with the ons lyinga the folds of the pleats.The pleated n both paralleand antiparallel forms.In the parallel Bpleated sheet,adjacent chains run in the same direction (NC or CN) In the antiparallel pleated sheet,adjacent strands run in opposite directions.Parallel-sheets tend to be more regular than antiparallel -sheets. Fig.2-24 A"pleated sheet"of paper with an antiparallel-sheet drawn on it. 2.4.5 The B-tur to bend,tu,and reorient itself to produce the required compact,globular structures. A simpl structure observed in many proteins is the &turn (also known as the tight turn or bend).in which the peptide chain forms a tight loop with the carbonyl oxygen of one residue hydrogen-bonded with the amide proton of the residue three positions down the chain.This H bond makes the turn a relatively stable structure.As shown in Figure2-25,theu allows the protein to reverse the direction of its peptide chain.This figure shows the wo major typeso B-turns,but a number of less common types are also found in protein structures.Certain amino acids,such as proline and glycine,occur frequently in Bturn sequences,and the particular conformation of the Bturn sequence depends to some extent on the amino acids composing it. 27
27 Additionally there are several other far less common types of helices found in proteins. The most common of these is the 310 helix, which contains 3.0 residues per turn (with 10 atoms in the ring formed by making the hydrogen bond three residues up the chain). It normally extends over shorter stretches of sequence than the α-helix. Other helical structures include the 27 ribbon and the π-helix, which has 4.4 residues and 16 atoms per turn and is thus called the 4.416 helix. 2.4.4 The β-pleated sheet Another type of structure commonly observed in proteins also forms because of local, cooperative formation of hydrogen bonds. That is the pleated sheet, or β-structure, often called the β-pleated sheet. This structure was also first postulated by Pauling and Corey in 1951 and has now been observed in many natural proteins. A -pleated sheet can be visualized by laying thin, pleated strips of paper side by side to make a “pleated sheet” of paper (Figure 2-24). Each strip of paper can then be pictured as a single peptide strand in which the peptide backbone makes a zigzag pattern along the strip, with the α-carbons lying at the folds of the pleats. The pleated sheet can exist in both parallel and antiparallel forms. In the parallel β-pleated sheet, adjacent chains run in the same direction (N → C or C → N). In the antiparallel β-pleated sheet, adjacent strands run in opposite directions. Parallel -sheets tend to be more regular than antiparallel -sheets. Fig. 2-24 A “pleated sheet” of paper with an antiparallel -sheet drawn on it. 2.4.5 The β-turn Most proteins are globular structures. The polypeptide chain must therefore possess the capacity to bend, turn, and reorient itself to produce the required compact, globular structures. A simple structure observed in many proteins is the β-turn (also known as the tight turn or β-bend), in which the peptide chain forms a tight loop with the carbonyl oxygen of one residue hydrogen-bonded with the amide proton of the residue three positions down the chain. This H bond makes the β-turn a relatively stable structure. As shown in Figure 2-25, the β-turn allows the protein to reverse the direction of its peptide chain. This figure shows the two major types of β-turns, but a number of less common types are also found in protein structures. Certain amino acids, such as proline and glycine, occur frequently in β-turn sequences, and the particular conformation of the β-turn sequence depends to some extent on the amino acids composing it

Due to the absence of a side chain,glycine is sterically the most adaptable of the amino acids,and it accommodates conveniently to other steric constraints in.Proline.however,has a cyclic struct and a fixedangle,so to some extent,it forces the fo -tum,and in many cases this facilitates the turning of a polypeptide chain upon itself.Such bends promote formation of antiparallel pleated sheets. Fig.2-25 The structures of two kinds of-turns (also called tight turns or Bbends) 2.46Super-secondary structure Supersecondary structures,also called motifs or simply folds,are particularly stable arrangements of several elements of secondary structure and the connections between them.There is no universal agreement among biochemists on the application of the three terms.and they are often used interchangeably.Theterms are also applied toa wide range of structures.Recognized motifs range from simple to appearing in repeating unitsorcombinations.A single large motif may comprise the entire protein.We have already encountered one well-studied motif,the coiled coil of -keratin,also found in a number of other proteins. Super-secondary structure can be produced by combinations of a-helix and pleated sheets ne isunit,two sucessive separated by a nonhelical segmentbec me enmeshes because compatible sie chains sheet are connected by polar amino acids and glycines to effect an abrupt change in direction of the polypeptide chain,call reverse or Bturns.The last one is Bap unit,two parallel Bpleated sheets are connected by an a-helix segment. Table 26 Secondary Structures and Properties of Fibrous Proteins Structure Characteristics Examples of occurrence a Helix Tough.insoluble protective a-Keratin of hair,feathers, cross-linked by structures of varying hardness and nails disulfide bonds and flexibility Soft,flexible filam Silk fibroin Collagen triple helix High tensile strength,without Collagen of tendons,bone stretch matrix 2.4.7 Fibrous Proteins 28
28 Due to the absence of a side chain, glycine is sterically the most adaptable of the amino acids, and it accommodates conveniently to other steric constraints in the β-turn. Proline, however, has a cyclic structure and a fixed φ angle, so, to some extent, it forces the formation of a -turn, and in many cases this facilitates the turning of a polypeptide chain upon itself. Such bends promote formation of antiparallel β-pleated sheets. Fig. 2-25 The structures of two kinds of -turns (also called tight turns or β-bends) 2.4.6 Super-secondary structure Supersecondary structures, also called motifs or simply folds, are particularly stable arrangements of several elements of secondary structure and the connections between them. There is no universal agreement among biochemists on the application of the three terms, and they are often used interchangeably. The terms are also applied to a wide range of structures. Recognized motifs range from simple to complex, sometimes appearing in repeating units or combinations. A single large motif may comprise the entire protein. We have already encountered one well-studied motif, the coiled coil of _-keratin, also found in a number of other proteins. Super-secondary structure can be produced by combinations of α-helix and β-pleated sheets. One is αα unit, two successive α-helices separated by a loop or nonhelical segment become enmeshes because of compatible side chains. The second is β-meander, two antiparallel β-pleated sheet are connected by polar amino acids and glycines to effect an abrupt change in direction of the polypeptide chain, call reverse or β-turns. The last one is βαβ unit, two parallel β-pleated sheets are connected by an α-helix segment. Table 2–6 Secondary Structures and Properties of Fibrous Proteins Structure Characteristics Examples of occurrence α Helix, cross-linked by disulfide bonds Tough, insoluble protective structures of varying hardness and flexibility α-Keratin of hair, feathers, and nails β Conformation Soft, flexible filaments Silk fibroin Collagen triple helix High tensile strength, without stretch Collagen of tendons, bone matrix 2.4.7 Fibrous Proteins