
Absolute configurations of all other carbon-based molecules are referenced to D.and the L configuration.Amino acids of the D configuration are nonetheless found in nature especially as components of certain peptide antibiotics,such as valinomycin,gramicidin,and actinomycin D.and in the cell walls of certain microorganisms.In spite of its widespread acceptance,problems exist with the D.L system of nomenclature.For example,this system can be r molecules with tw nore chiral centers.To address proble S, the (R S system of nomenclature for chiral molecules was proposed in 1956 by Robert Cahn,Sir Christopher Ingold,and Vladimir Prelog.In this more versatile system,priorities are assigned to each of the groups attached to a chiral center on the basis of atomic number,atoms with higher atomic numbers having higher priorities (see the box,page 100).The newer (R.S)system of nomenclature is superior to the older D.Lsystem in one important way CHO CHO HO- C-H H-C-OH CH OH CHOH L-Glyceraldehyde p-Glyceraldehyde COOH COOH HN-C一H H-C一NHg CH,OH L-Serine D-Serine Fig.2-5 The configuration of the common Lamino acids can be related to the configuration of L(-)-glyceraldehyde as shown.These drawings are knowr as Fischer projections.The horizontal lines of the Fischer projections are meant to indicate bonds coming out of the page from the central carbon,and vertical lines represent bonds extending behind the page from the central carbon atom 223.2 Spectroscopic properties of amino acids One of the most important and exciting advances in modern biochemistry has been the application of spectroscopic methods.which measure the absorption and emission of energy of different frequencies by molecules and atoms.Spectroscopic studies of proteins,uceicacids,and th structure and dynamic thes e molecules Ultraviolet Spectra Many details of the structure and chemistry of the amino acids have been elucidated or at least confirmed by spectroscopic measurements.None of the amino acids absorbs light in the visible region of the electromagnetic spectrum.Several of the amino acids,however
9 Absolute configurations of all other carbon-based molecules are referenced to D- and L-glyceraldehyde. When sufficient care is taken to avoid racemization of the amino acids during hydrolysis of proteins, it is found that all of the amino acids derived from natural proteins are of the L configuration. Amino acids of the D configuration are nonetheless found in nature, especially as components of certain peptide antibiotics, such as valinomycin, gramicidin, and actinomycin D, and in the cell walls of certain microorganisms. In spite of its widespread acceptance, problems exist with the D,L system of nomenclature. For example, this system can be ambiguous for molecules with two or more chiral centers. To address such problems, the (R,S ) system of nomenclature for chiral molecules was proposed in 1956 by Robert Cahn, Sir Christopher Ingold, and Vladimir Prelog. In this more versatile system, priorities are assigned to each of the groups attached to a chiral center on the basis of atomic number, atoms with higher atomic numbers having higher priorities (see the box, page 100). The newer (R,S) system of nomenclature is superior to the older D,L system in one important way. Fig. 2-5 The configuration of the common L-amino acids can be related to the configuration of L(-)-glyceraldehyde as shown. These drawings are known as Fischer projections.The horizontal lines of the Fischer projections are meant to indicate bonds coming out of the page from the central carbon, and vertical lines represent bonds extending behind the page from the central carbon atom. 2.2.3.2 Spectroscopic properties of amino acids One of the most important and exciting advances in modern biochemistry has been the application of spectroscopic methods, which measure the absorption and emission of energy of different frequencies by molecules and atoms. Spectroscopic studies of proteins, nucleic acids, and other biomolecules are providing many new insights into the structure and dynamic processes in these molecules. Ultraviolet Spectra Many details of the structure and chemistry of the amino acids have been elucidated or at least confirmed by spectroscopic measurements. None of the amino acids absorbs light in the visible region of the electromagnetic spectrum. Several of the amino acids, however
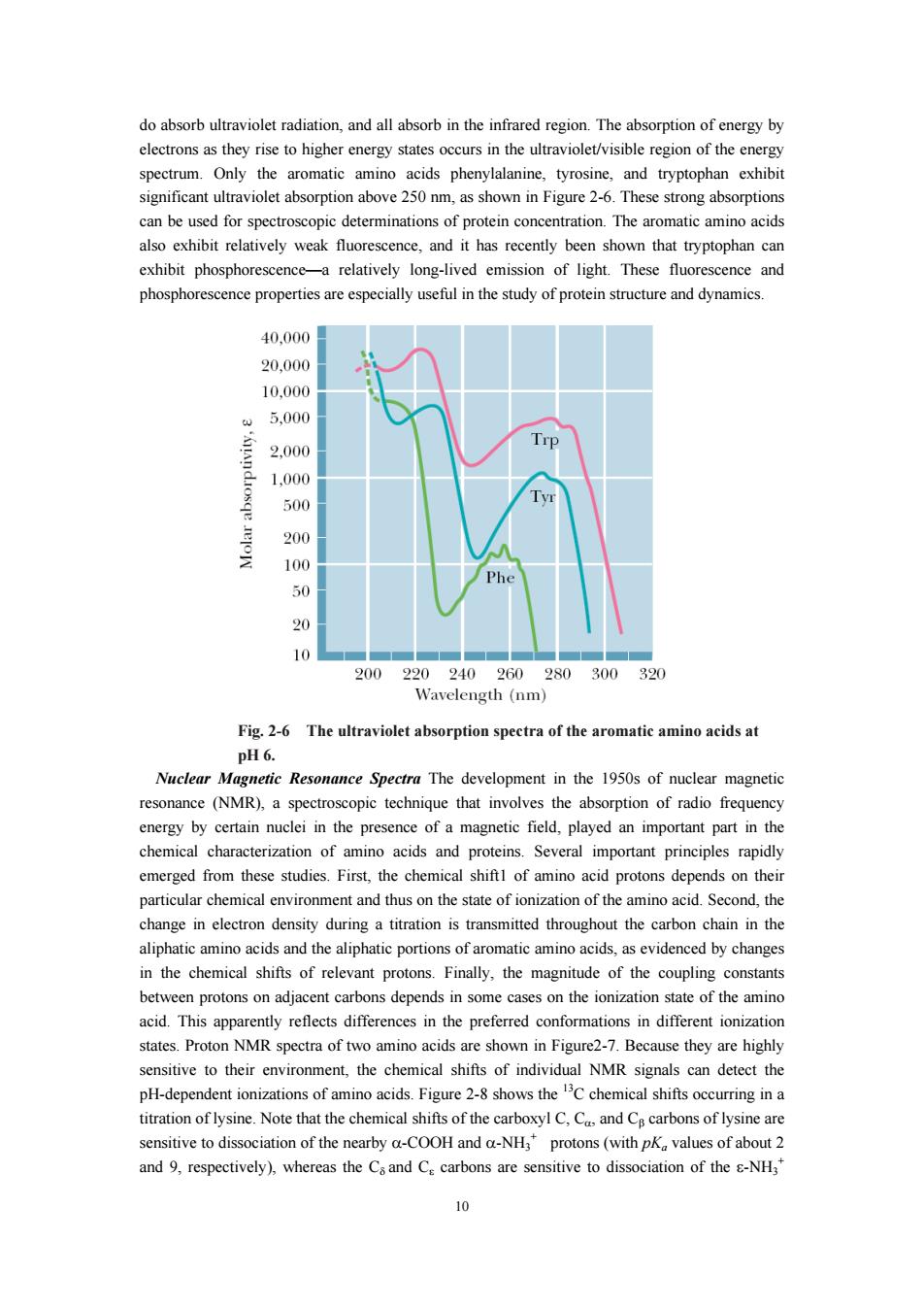
do absorb ultraviolet radiation,and all absorb in the infrared region.The absorption of energy by thevrery states occurs in the ultraviolet/visible regiono spectrum Only min acids and tryptopha significant ultraviolet absorption above 250m,as shown in Figure 2-6 These strong absorption can be used for spectroscopic determinations of protein concentration.The aromatic amino acids also exhibit relatively weak fluorescence,and it has recently been shown that tryptophan can exhibit phosphorescence-a relatively long-lived emission of light.These fluorescence and phosphorescence pr rties are useful in the study of proteinstructure and dynamics 40,000 20.000 10.000 5,00 2,00 Trp 1.000 500 20 100 50 20 200 240 260 280300 32 Wavelength(nm Fig.2-6 The ultraviolet absorption spectra of the aromatic amino acids at pH6. Nuclear Magnetie Resonance Spectra The development in the 1950s of nuclear magnetic resonance (NMR),a spectroscopic technique that involves the absorption of radio freque in the chemical of amn acids and proteins.Several important principles rapid emerged from these studies.First,the chemical shiftl of amino acid protons depends on their particular chemical environment and thus on the state of ionization of the amino acid.Second,the change in electron density during a titration is transmitted throughout the carbon chain in the aliphatic aminoacids and the aiphatic portions of aromaticaminoacids as evidenced by changes in the chemical shifts of relevant protons.Finally,the magnitude of the coupling constant between protons on adjacent carbons depends in some cases on the ionization state of the amino acid.This apparently reflects differences in the preferred conformations in different ionization states.Proton NMR spectra of two amino acids are shown in Figure2-7.Because they are highly sensitive to their enviro ment,the chemical shifts of individual NMR signals can detect the H-dependent ion titration of lysine.Note that the chemical shifts of the carboxyl C,C,and C carbons of lysine are sensitive to dissociation of the nearby a-COOH and a-NH,protons(with pK values of about 2 and 9,respectively),whereas the Csand C carbons are sensitive to dissociation of the -NH3
10 do absorb ultraviolet radiation, and all absorb in the infrared region. The absorption of energy by electrons as they rise to higher energy states occurs in the ultraviolet/visible region of the energy spectrum. Only the aromatic amino acids phenylalanine, tyrosine, and tryptophan exhibit significant ultraviolet absorption above 250 nm, as shown in Figure 2-6. These strong absorptions can be used for spectroscopic determinations of protein concentration. The aromatic amino acids also exhibit relatively weak fluorescence, and it has recently been shown that tryptophan can exhibit phosphorescence—a relatively long-lived emission of light. These fluorescence and phosphorescence properties are especially useful in the study of protein structure and dynamics. Fig. 2-6 The ultraviolet absorption spectra of the aromatic amino acids at pH 6. Nuclear Magnetic Resonance Spectra The development in the 1950s of nuclear magnetic resonance (NMR), a spectroscopic technique that involves the absorption of radio frequency energy by certain nuclei in the presence of a magnetic field, played an important part in the chemical characterization of amino acids and proteins. Several important principles rapidly emerged from these studies. First, the chemical shift1 of amino acid protons depends on their particular chemical environment and thus on the state of ionization of the amino acid. Second, the change in electron density during a titration is transmitted throughout the carbon chain in the aliphatic amino acids and the aliphatic portions of aromatic amino acids, as evidenced by changes in the chemical shifts of relevant protons. Finally, the magnitude of the coupling constants between protons on adjacent carbons depends in some cases on the ionization state of the amino acid. This apparently reflects differences in the preferred conformations in different ionization states. Proton NMR spectra of two amino acids are shown in Figure2-7. Because they are highly sensitive to their environment, the chemical shifts of individual NMR signals can detect the pH-dependent ionizations of amino acids. Figure 2-8 shows the 13C chemical shifts occurring in a titration of lysine. Note that the chemical shifts of the carboxyl C, Cα, and Cβ carbons of lysine are sensitive to dissociation of the nearby α-COOH and α-NH3 + protons (with pKa values of about 2 and 9, respectively), whereas the Cδ and Cε carbons are sensitive to dissociation of the ε-NH3 +
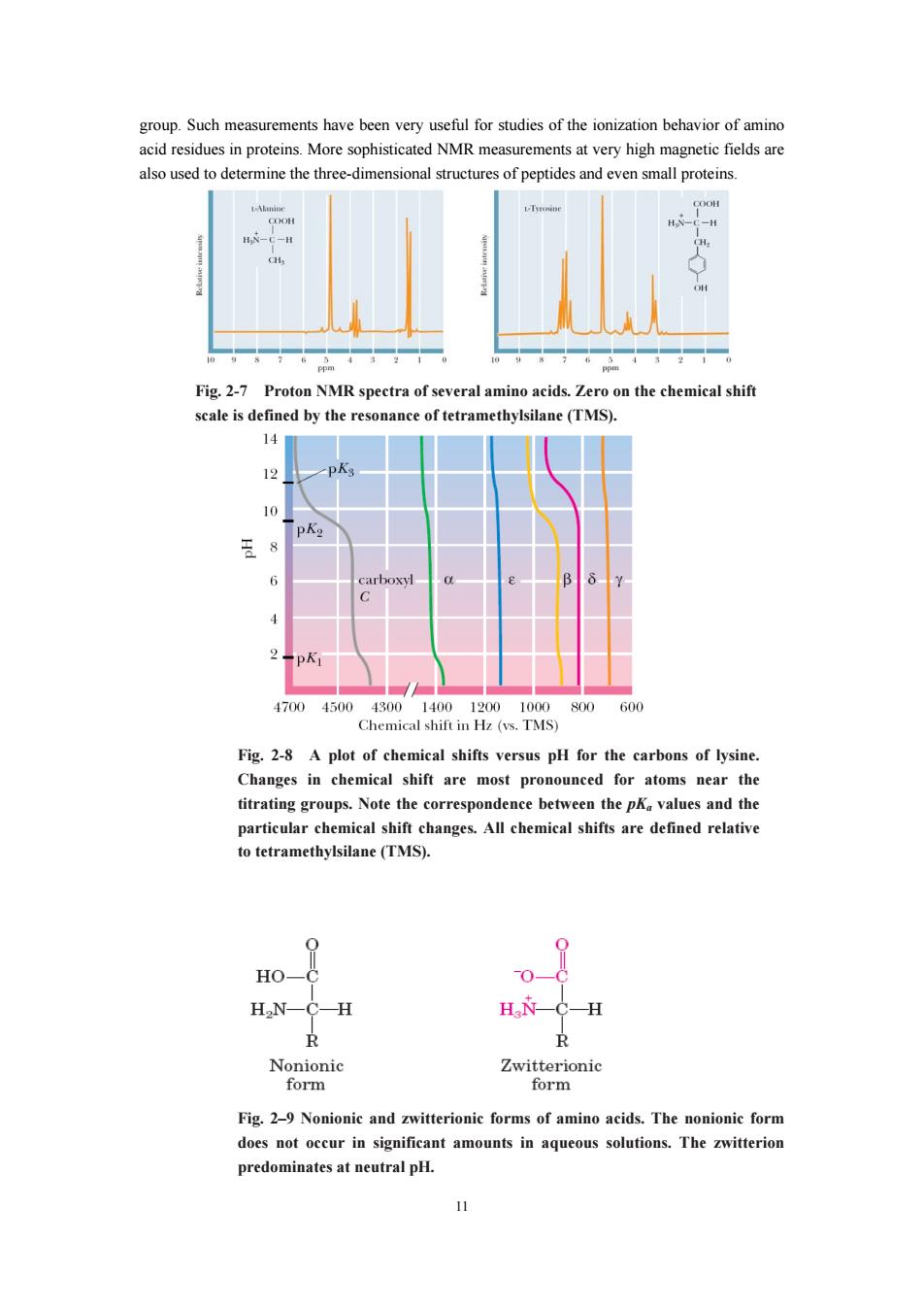
also used to determine the three-dimensionals structures of peptides and even small proteins Fig.2-7 Proton NMR spectra of several amino acids.Zero on the chemical shift cale is defined by the reso nce of tetramethylsilane(TMS). 14 12 PA 王8 1700450 0.TMS 800 60 Fig.2-8 A plot of chemical shifts versus pH for the carbons of lysine Changes in chemical shift are most pronounced for atoms near the titrating g oups.Note the correspondence between the p values and the particular cher ical changes.All chemical shifts are defined relativ to tetramethylsilane (TMS). 0 H0一 2 H2N-C-H H衣-C-H R Nonionic Zwitterionic form form Fig.2-9 Nonionic and zwitterionic forms of amino acids.The nonionic form does not occur in significant amounts in aqueous solutions.The zwitterion predominates at neutral pH
11 group. Such measurements have been very useful for studies of the ionization behavior of amino acid residues in proteins. More sophisticated NMR measurements at very high magnetic fields are also used to determine the three-dimensional structures of peptides and even small proteins. Fig. 2-7 Proton NMR spectra of several amino acids. Zero on the chemical shift scale is defined by the resonance of tetramethylsilane (TMS). Fig. 2-8 A plot of chemical shifts versus pH for the carbons of lysine. Changes in chemical shift are most pronounced for atoms near the titrating groups. Note the correspondence between the pKa values and the particular chemical shift changes. All chemical shifts are defined relative to tetramethylsilane (TMS). Fig. 2–9 Nonionic and zwitterionic forms of amino acids. The nonionic form does not occur in significant amounts in aqueous solutions. The zwitterion predominates at neutral pH
22 33 As acid and bases of amino acids When an amin (German for"hybrid ion"),shown in Figure 3-9.A zwitterion can act as either an acid (proton donor): H H R-0-C0O-R-0-C0O-+H +NH NH2 Zwitterion ora base(proton acceptor): H H R-C-COO-+H*=R-C-COOH 2 +NH Substances having e f called (from olytes").Asimple ylic am ino acid is a diprotic acid when fully protonate -it has two groups,the-COOH group and the-NH group,that can yield protons: H H+ H R-CC0H→RC-C00-CC00 Net +NH +NH NH charge:+1 0 Consider the acid-base behavior of glycine,the simplest amino acid.Figure 2-10 shows the titration curve of the diprotie form of glycine.The plot has two distinct stages,corresponding to o stages mbles in shap titration curve of a monoprotic acid,such as acetic acid,and can be analyzed in the same way.At very low pH.the predominant ionic species of glycine is the fully protonated form.H:N-CH,- COOH.At the midpoint in the first stage of the titration,in which the -COOH group of glycine loses its proton,equimolar concentrations of the proton-donor (HN-CH2-COOH)and proton tor CH 1-CH、-CO0 species are pre oup being titrate For glycine,the pH at the midpoint is 2.34,thus its OCOOH group has a pKa (labeled pKI in Fig 2-10)of 2.34.As the titration proceeds,another important point is reached at pH 5.97.Here there is another point of inflection.at which removal of the first proton is essentially complete and removal of the second has just begun.At this pHglycine is y as the dipolari ion "HaN CH:-COO sha o the significance of this point in the titration cur (labeled pl in Fig.2-10)shortly.The second stage of the titration corresponds to the removal of a proton from the-NHs'group of glycine.The pH at the midpoint of this stage is 9.60,equal to the 12
12 2.2.3.3 As acid and bases of amino acids When an amino acid is dissolved in water, it exists in solution as the dipolar ion, or zwitterion (German for “hybrid ion”), shown in Figure 3–9. A zwitterion can act as either an acid (proton donor): or a base (proton acceptor): Substances having this dual nature are amphoteric and are often called ampholytes (from “amphoteric electrolytes”). A simple monoamino monocarboxylic α-amino acid, such as alanine, is a diprotic acid when fully protonated—it has two groups, the -COOH group and the -NH3 + group, that can yield protons: Consider the acid–base behavior of glycine, the simplest amino acid. Figure 2–10 shows the titration curve of the diprotic form of glycine. The plot has two distinct stages, corresponding to deprotonation of two different groups on glycine. Each of the two stages resembles in shape the titration curve of a monoprotic acid, such as acetic acid, and can be analyzed in the same way. At very low pH, the predominant ionic species of glycine is the fully protonated form, + H3N-CH2- COOH. At the midpoint in the first stage of the titration, in which the -COOH group of glycine loses its proton, equimolar concentrations of the proton-donor (+ H3N-CH2-COOH) and proton-acceptor (+ H3N-CH2-COO- ) species are present. At the midpoint of any titration, a point of inflection is reached where the pH is equal to the pKa of the protonated group being titrated. For glycine, the pH at the midpoint is 2.34, thus its OCOOH group has a pKa (labeled pK1 in Fig. 2–10) of 2.34. As the titration proceeds, another important point is reached at pH 5.97. Here there is another point of inflection, at which removal of the first proton is essentially complete and removal of the second has just begun. At this pH glycine is present largely as the dipolar ion + H3N -CH2-COO- . We shall return to the significance of this inflection point in the titration curve (labeled pI in Fig. 2–10) shortly. The second stage of the titration corresponds to the removal of a proton from the -NH3 + group of glycine. The pH at the midpoint of this stage is 9.60, equal to the
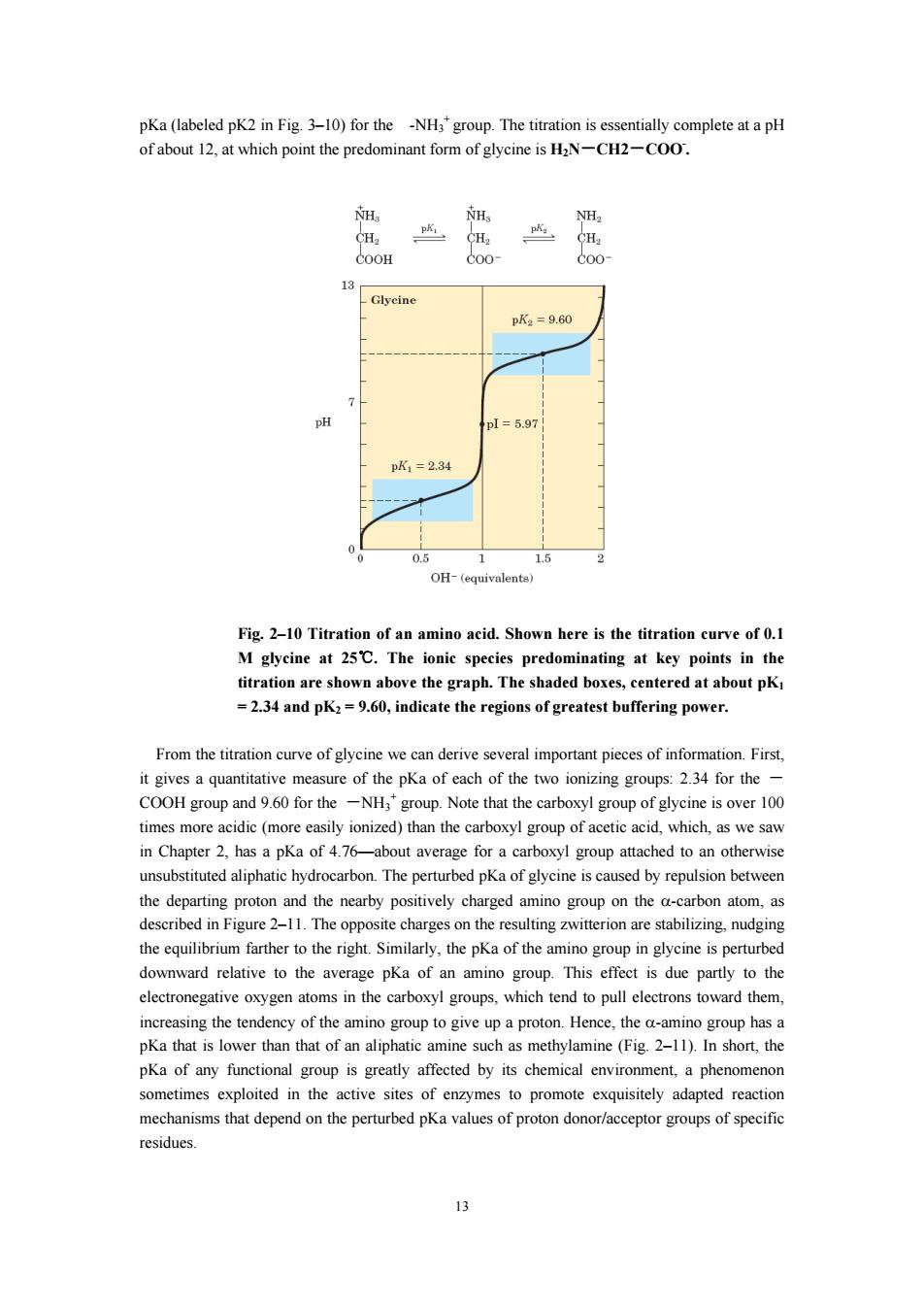
pKa(labeled pK2 in Fig.3-10)for the -NH'group.The titration is essentially complete at a pH of about which point the predominant form of glycine is HaN-CH2-COO. 13 pK2=9.60 p1=5.97 pK1=234 0.5 2 OH-(equivalenta) Fig.2-10 Titration of an amino acid.Shown here is the titration curve of 0.1 abo ve the graph.The sh .dK:0,indicate the of greatest bufering power. From the titration curve of glycine we can derive several important pieces of information.First. it gives a quantitative measure of the pKa of each of the two ionizing groups:2.34 for the- COOH group for the-NHgroup.Note that the carboxyl gro of glycine is over 100 time es more ac c(m reeasily ionized)than the carboxyl group of aceticac which,as we sav in Chapter 2,has a pKa of 4.76-about average for a carboxyl group attached to an otherwise unsubstituted aliphatic hydrocarbon.The perturbed pKa of glycine is caused by repulsion between the departing proton and the nearby positively charged amino group on the a-carbon atom.as described in Figure 2-11 The o charge s on the resulting witterion are stabilizing.nudgi Similarly,the pKa of the amino group n cine is perturb downward relative to the average pKa of an amino group.This effect is due partly to the electronegative oxygen atoms in the carboxyl groups.which tend to pull electrons toward them. increasing the tendency of the amino group to give up a proton.Hence,the a-amino group has a pKa that is lower than that of an aliphatic amine such as methylamine(Fig 2-11).In short,the pKa of any functi oup tly affected by its chemical nviron sometimes exploited in the active sites of enzymes to promote exquisitely adapted reactic mechanisms that depend on the perturbed pKa values of proton donor/acceptor groups of specific residues. 13
13 pKa (labeled pK2 in Fig. 3–10) for the -NH3 + group. The titration is essentially complete at a pH of about 12, at which point the predominant form of glycine is H2N-CH2-COO- . Fig. 2–10 Titration of an amino acid. Shown here is the titration curve of 0.1 M glycine at 25℃. The ionic species predominating at key points in the titration are shown above the graph. The shaded boxes, centered at about pK1 = 2.34 and pK2 = 9.60, indicate the regions of greatest buffering power. From the titration curve of glycine we can derive several important pieces of information. First, it gives a quantitative measure of the pKa of each of the two ionizing groups: 2.34 for the - COOH group and 9.60 for the -NH3 + group. Note that the carboxyl group of glycine is over 100 times more acidic (more easily ionized) than the carboxyl group of acetic acid, which, as we saw in Chapter 2, has a pKa of 4.76—about average for a carboxyl group attached to an otherwise unsubstituted aliphatic hydrocarbon. The perturbed pKa of glycine is caused by repulsion between the departing proton and the nearby positively charged amino group on the α-carbon atom, as described in Figure 2–11. The opposite charges on the resulting zwitterion are stabilizing, nudging the equilibrium farther to the right. Similarly, the pKa of the amino group in glycine is perturbed downward relative to the average pKa of an amino group. This effect is due partly to the electronegative oxygen atoms in the carboxyl groups, which tend to pull electrons toward them, increasing the tendency of the amino group to give up a proton. Hence, the α-amino group has a pKa that is lower than that of an aliphatic amine such as methylamine (Fig. 2–11). In short, the pKa of any functional group is greatly affected by its chemical environment, a phenomenon sometimes exploited in the active sites of enzymes to promote exquisitely adapted reaction mechanisms that depend on the perturbed pKa values of proton donor/acceptor groups of specific residues