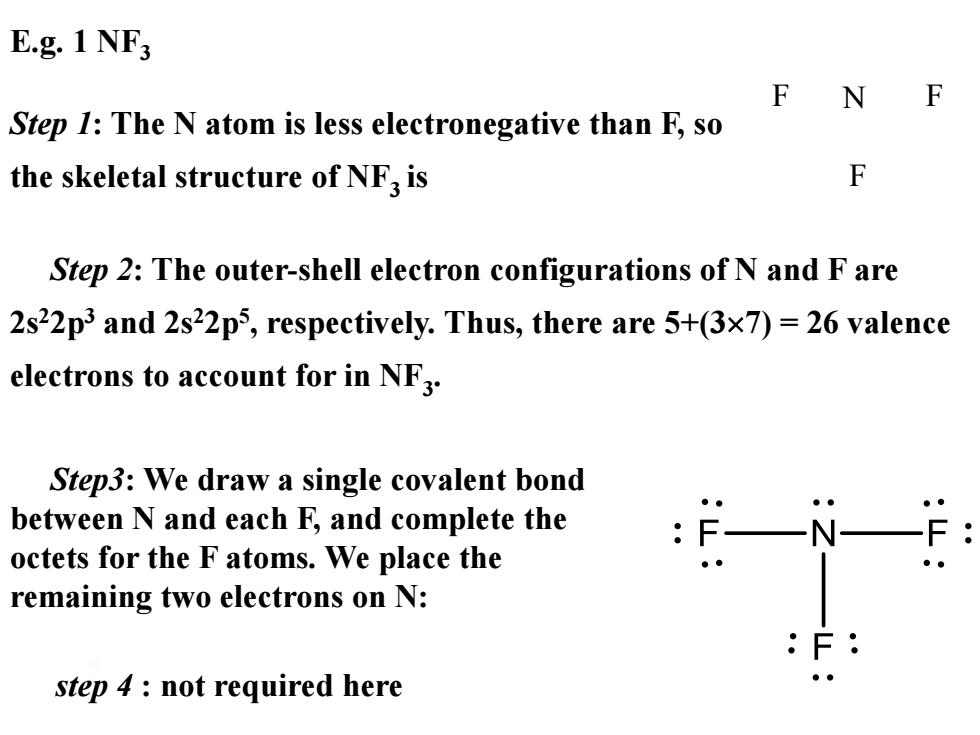
E.g.1 NF F N F Step 1:The N atom is less electronegative than F,so the skeletal structure of NF3 is F Step 2:The outer-shell electron configurations of N and F are 2s22p3 and 2s22p5,respectively.Thus,there are 5+(3x7)=26 valence electrons to account for in NF3. Step3:We draw a single covalent bond between N and each F,and complete the octets for the F atoms.We place the remaining two electrons on N: step 4 not required here ●●
E.g. 1 NF3 Step 1: The N atom is less electronegative than F, so the skeletal structure of NF3 is F N F F Step3: We draw a single covalent bond between N and each F, and complete the octets for the F atoms. We place the remaining two electrons on N: Step 2: The outer-shell electron configurations of N and F are 2s22p3 and 2s22p5 , respectively. Thus, there are 5+(37) = 26 valence electrons to account for in NF3 . step 4 : not required here
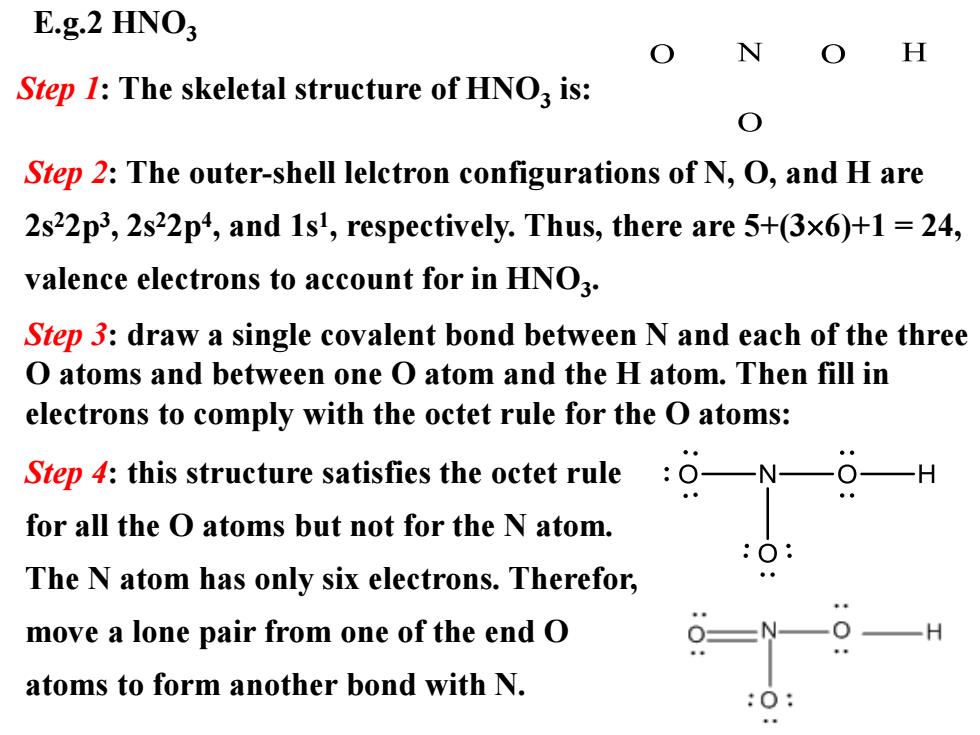
E.g.2 HNO H Step 1:The skeletal structure of HNO is: Step 2:The outer-shell lelctron configurations of N,O,and H are 2s22p3,2s22p4,and 1sl,respectively.Thus,there are 5+(3x6)+1 24, valence electrons to account for in HNO3. Step 3:draw a single covalent bond between N and each of the three O atoms and between one O atom and the H atom.Then fill in electrons to comply with the octet rule for the O atoms: Step 4:this structure satisfies the octet rule O-N- for all the O atoms but not for the N atom. The N atom has only six electrons.Therefor, move a lone pair from one of the end O atoms to form another bond with N
E.g.2 HNO3 Step 1: The skeletal structure of HNO3 is: Step 2: The outer-shell lelctron configurations of N, O, and H are 2s22p3 , 2s22p4 , and 1s1 , respectively. Thus, there are 5+(36)+1 = 24, valence electrons to account for in HNO3 . Step 4: this structure satisfies the octet rule for all the O atoms but not for the N atom. The N atom has only six electrons. Therefor, move a lone pair from one of the end O atoms to form another bond with N. Step 3: draw a single covalent bond between N and each of the three O atoms and between one O atom and the H atom. Then fill in electrons to comply with the octet rule for the O atoms:

E.g.3 carbonate ion CO2 Step 1:deduce the skeletal structure of the carbonate ion by recognizing that C is less electronegative than O: 0 C Step 2:The outer-shell electron configurations of C and O are 2s22p2 and 2s22p4,respectively,and the ion itself has two negative charges.Thus,the total number of electrons is 4+(3X6)+2=24. Step 3:draw a single covalent bond between C and each O and comply with the octet rule for the O atoms::. Step 4:Although the octet rule is satisfied for the O atoms,it is not for the C atom. Therefore,move a lone pair from one of the O atoms to form another bond with C.The octet rule is also satisfied for the C atom:
Step 2: The outer-shell electron configurations of C and O are 2s22p2 and 2s22p4 , respectively, and the ion itself has two negative charges. Thus, the total number of electrons is 4+(3×6)+2 = 24. Step 4: Although the octet rule is satisfied for the O atoms, it is not for the C atom. Therefore, move a lone pair from one of the O atoms to form another bond with C. The octet rule is also satisfied for the C atom: E.g. 3 carbonate ion CO3 2- Step 1: deduce the skeletal structure of the carbonate ion by recognizing that C is less electronegative than O: Step 3: draw a single covalent bond between C and each O and comply with the octet rule for the O atoms:
Exceptions to the octet rule:BF3,PCls,SF et al B:2S22P1;F:2S22P5 The octet rule and Lewis structure do not present a complete picture of covalent bonding
Exceptions to the octet rule: BF3 , PCl5 , SF6 et al B: 2S22P1 ; F: 2S22P5 The octet rule and Lewis structure do not present a complete picture of covalent bonding

6.2 Valence Bond Theory -6.2.1 The Essence and Characteristics of Covalent Bond 6.2.2 The Types of Covalent Bond
6.2.1 The Essence and Characteristics of Covalent Bond §6.2 Valence Bond Theory 6.2.2 The Types of Covalent Bond