
§16 The elements of oxygen family 16.1 Outline on oxygen family 16.2 Oxygen and the related compounds 16.3 Sulfur and the related compounds
§16 The elements of oxygen family 16.3 Sulfur and the related compounds 16.2 Oxygen and the related compounds 16.1 Outline on oxygen family
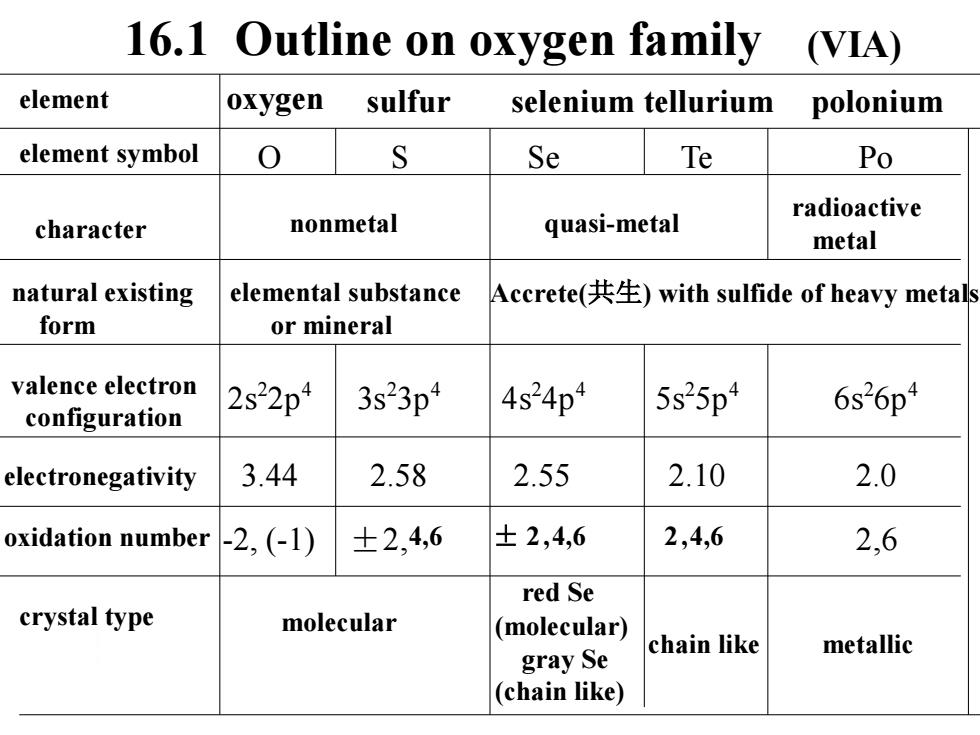
16.1 Outline on oxygen family (VIA) element oxygen sulfur selenium tellurium polonium element symbol 0 S Se Te Po radioactive character nonmetal quasi-metal metal natural existing elemental substance Accrete(共生)with sulfide of heavy metals form or mineral valence electron 2s22p4 3s23p4 4s24p4 5s25p4 6s26p4 configuration electronegativity 3.44 2.58 2.55 2.10 2.0 oxidation number -2,(-1) ±2,4,6 ±2,4,6 2,4,6 2,6 red Se crystal type molecular (molecular) chain like metallic gray Se (chain like)
16.1 Outline on oxygen family oxygen sulfur selenium tellurium polonium element symbol (VIA) O S Se Te Po character nonmetal quasi-metal radioactive metal natural existing form elemental substance or mineral Accrete(共生) with sulfide of heavy metals valence electron configuration 2s 2 2p4 3s2 3p4 4s 2 4p4 5s 2 5p4 6s 2 6p4 electronegativity 3.44 2.58 2.55 2.10 2.0 oxidation number -2, (-1) ±2,4,6 ± 2 ,4,6 2 ,4,6 2,6 crystal type molecular red Se (molecular) gray Se (chain like) chain like metallic element

Sulfur 夏威夷劳厄火山裂缝喷 天然硫晶体 气孔附近的天然疏沉积
夏威夷劳厄火山裂缝喷 气孔附近的天然硫沉积 天然硫晶体

Se Te Covalent hydride H2O H2S H2Se H,Te chemical reactivity: low high stability: high low acidity: weak strong mp and bp: the highest low high
H2O H2S H2Se H2Te chemical reactivity: low high stability: high low acidity: weak strong mp and bp: the highest low high Se Te Covalent hydride
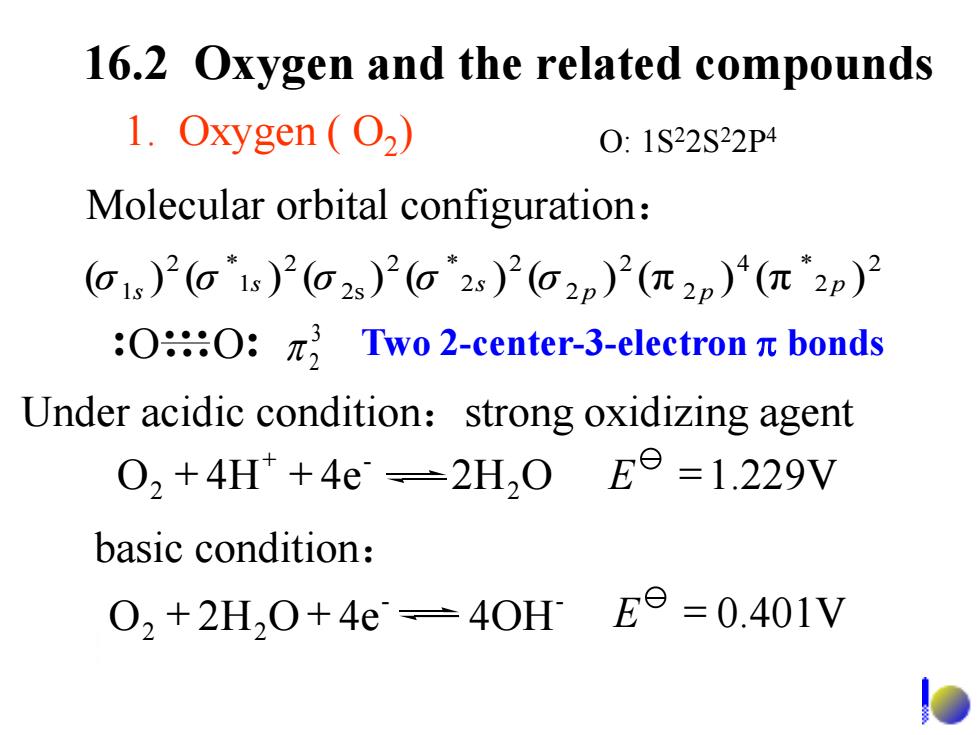
16.2 Oxygen and the related compounds 1.Oxygen (O2) 0:1S22S22P4 Molecular orbital configuration: G1s)2(G1s)2(o2s)2(G2s)2(G2p)2(π2p)4(π2p)2 :OO:πTwo2-center--3-electronπbonds Under acidic condition:strong oxidizing agent 02+4H+4e=2H,0E6=1.229V basic condition: 02+2H20+4e=40HEe=0.401V
basic condition: 2 2 4 2 2 2 2 2 2 2s 2 1 2 1 ( ) ( ) ( ) ( ) ( ) (π ) (π p ) * p p s * s * σ s σ σ σ σ 1. Oxygen ( O2 ) Molecular orbital configuration: 16.2 Oxygen and the related compounds O O Under acidic condition:strong oxidizing agent O 2H O 4e 4OH E = 0.401V - - 2 2 + + O 4H 4e 2H2O E =1.229V - 2 + + + 3 2 Two 2-center-3-electron bonds O: 1S22S22P4