
18 Hydrogen and the rare gases 8.1 The discovery of the rare gases 18.2 Properties and uses of the rare gases -18.3 The existence and recovery of the rare gases -18.4 The rare gas compounds -18.5 Summarry on the main group elements
18.1 The discovery of the rare gases §18 Hydrogen and the rare gases 18.4 The rare gas compounds 18.3 The existence and recovery of the rare gases 18.2 Properties and uses of the rare gases 18.5 Summarry on the main group elements

18.1 The discovery of the rare gases Rare gases:He Ne Ar Kr Xe Rn Valence e:ns2npo “第三位小数的胜利” fractionate N2 from air:1.2572 gL Ar Chemical preparation of N2:1.2505gL-1
18.1 The discovery of the rare gases Ar Rare gases:He Ne Ar Kr Xe Rn “第三位小数的胜利” fractionate N2 from air:1.2572 g•L-1 Chemical preparation of N2:1.2505g•L-1 Valence e-:ns 2np 6
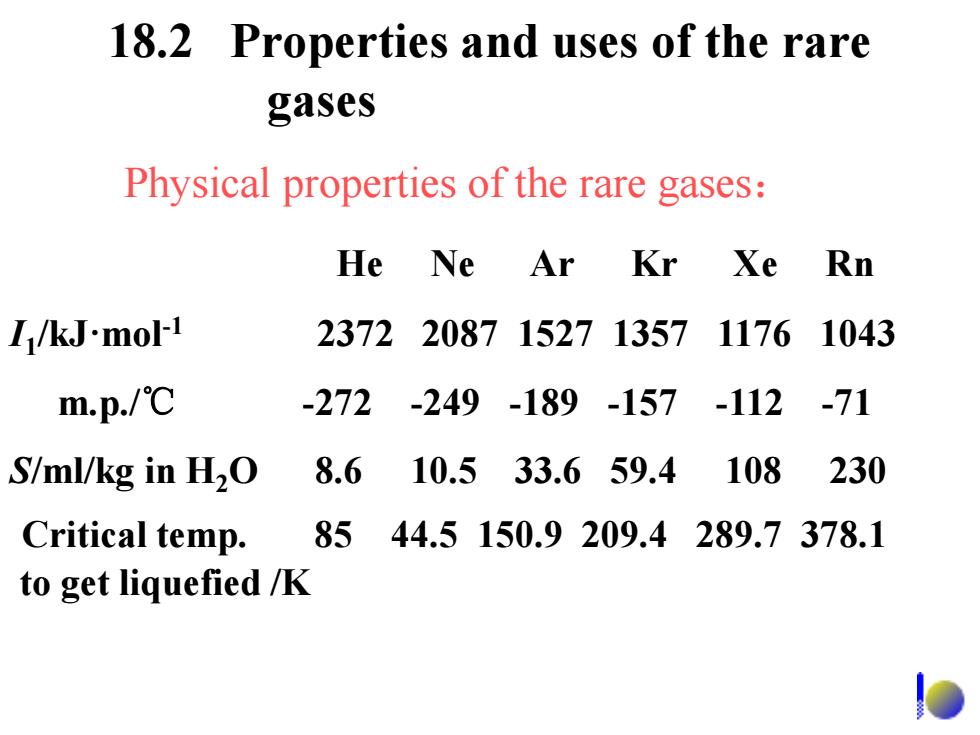
18.2 Properties and uses of the rare gases Physical properties of the rare gases: He Ne Ar Kr Xe Rn I/kJ-mol-1 237220871527135711761043 m.p./℃ -272-249-189-157-112 -71 S/ml/kg in H2O 8.6 10.533.659.4 108 230 Critical temp. 8544.5150.9209.4289.7378.1 to get liquefied /K
18.2 Properties and uses of the rare gases He Ne Ar Kr Xe Rn I1 /kJ·mol-1 2372 2087 1527 1357 1176 1043 m.p./℃ -272 -249 -189 -157 -112 -71 S/ml/kg in H2O 8.6 10.5 33.6 59.4 108 230 Physical properties of the rare gases: to get liquefied /K Critical temp. 85 44.5 150.9 209.4 289.7 378.1
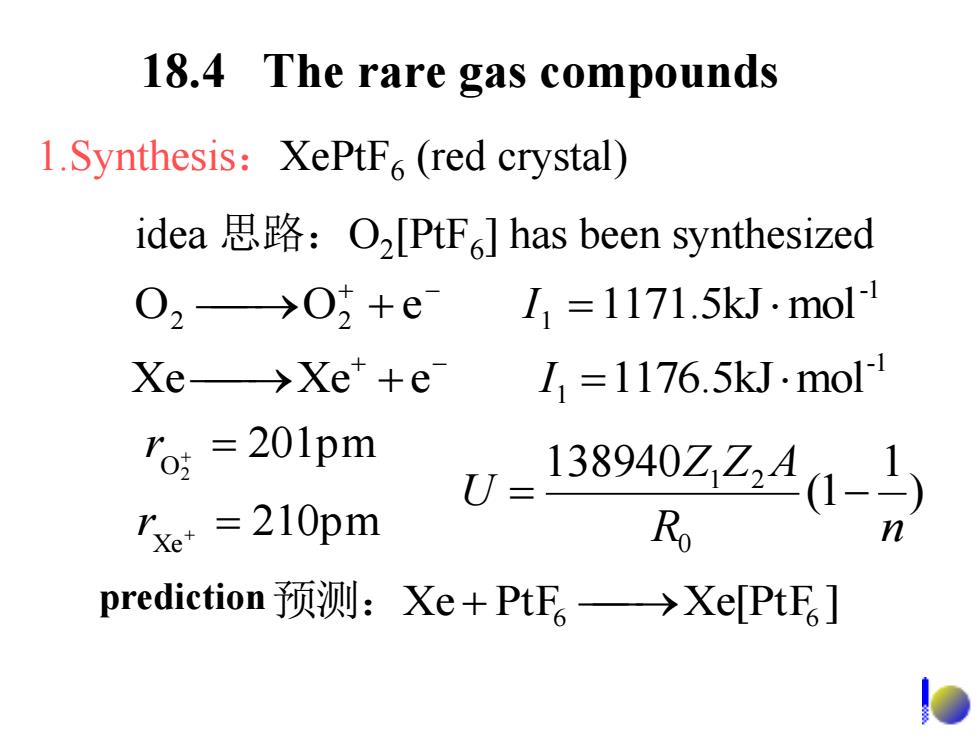
18.4 The rare gas compounds 1.Synthesis:XePtF(red crystal) idea思路:O2[PtF6]has been synthesized 02→0+eL1=1171.5 kJ.mol Xe→Xe+e 11=1176.5kJ.mol 'o=201pm 138940Z,Z41-〉 rxe =210pm R prediction预测:Xe+PtF。→Xe[PtE。]
18.4 The rare gas compounds 1.Synthesis:XePtF6 (red crystal) idea 思路:O2 [PtF6 ] has been synthesized Xe PtF Xe[PtF ] 预测: 6 6 ) 1 (1 138940 0 1 2 R n Z Z A U r Xe 210pm 201pm O2 r -1 Xe Xe e 1 1176.5kJmol I -1 O2 O2 e 1 1171.5kJmol I prediction

2.Molecular structure VSEPR theory: XeOF,V.P.-(8+0+4)-6 squarepyramidal 2 XeF,V.P.)7distroted octahedral Question:Describe the molecular shape of XeF,and XeF2
2. Molecular structure VSEPR theory: (8 6) 7 distrotedoctahedral 2 1 XeF V.P. (8 0 4) 6 square pyramidal 2 1 XeOF V.P. 6 4 Question:Describe the molecular shape of XeF4 and XeF2 ?