
§17 The halogens 17.1 The halogens 17.2 The elemental substances 17.3 Hydrogen halides 17.4 Halides and Polyhalides 17.5 halogen oxyacids
§17 The halogens 17.5 halogen oxyacids 17.4 Halides and Polyhalides 17.3 Hydrogen halides 17.2 The elemental substances 17.1 The halogens
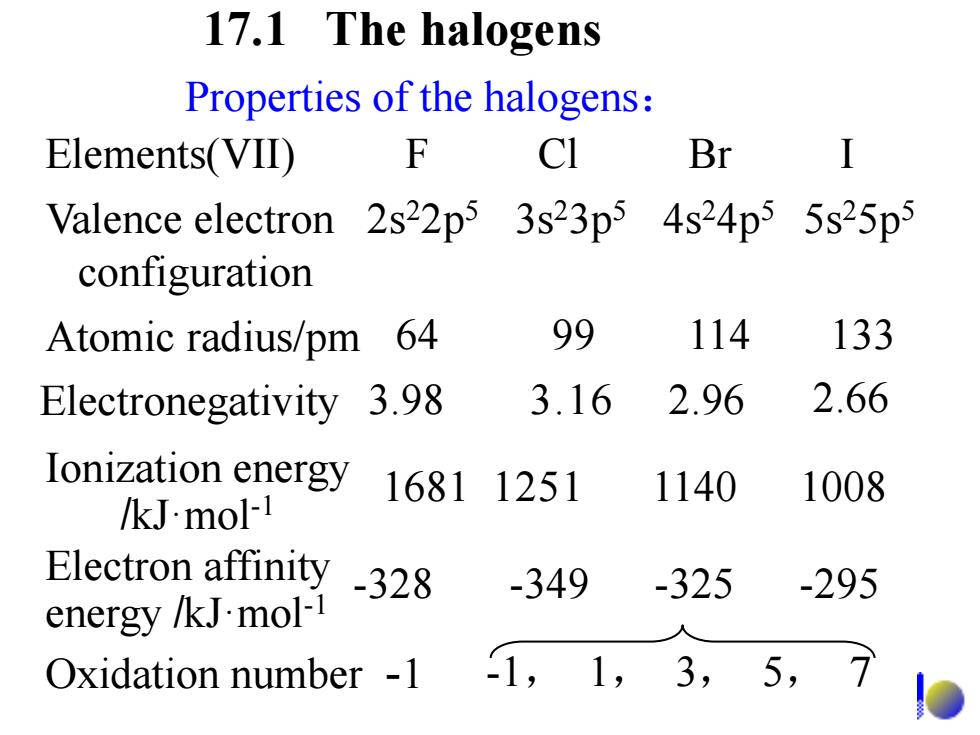
17.1 The halogens Properties of the halogens: Elements(VID) F CI Br I Valence electron 2s22p5 3s23p5 4s24p5 5s25p5 configuration Atomic radius/pm 64 99 114 133 Electronegativity 3.98 3.16 2.96 2.66 Ionization energy 16811251 1140 1008 /kJ.mol-1 Electron affinity -328 -349 -325 -295 energy /kJ.mol-1 Oxidation number -1 1,1,3,5,7
Properties of the halogens: Elements(VII) F Cl Br I Valence electron 2s22p5 3s23p5 4s24p5 5s25p5 configuration Atomic radius/pm 64 99 114 133 Ionization energy /kJ·mol-1 1681 1251 1140 1008 Electronegativity 3.98 3.16 2.96 2.66 Electron affinity energy /kJ·mol-1 -328 -349 -325 -295 Oxidation number -1 -1, 1, 3, 5, 7 17.1 The halogens
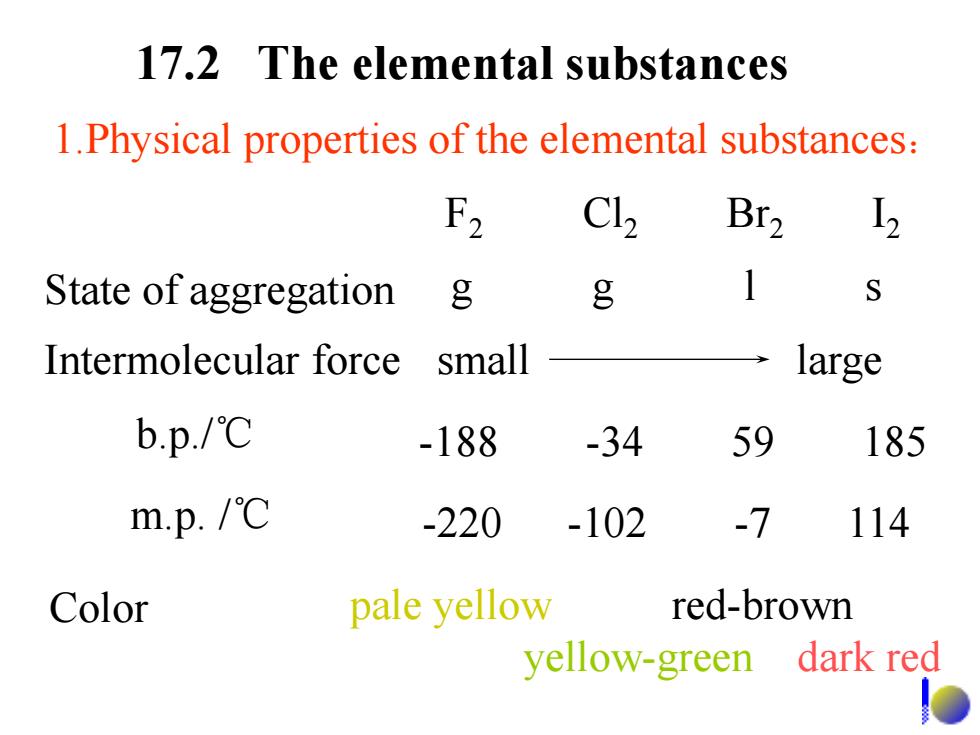
17.2 The elemental substances 1.Physical properties of the elemental substances: F2 C12 Br2 12 State of aggregation g g Intermolecular force small large b.p./℃ -188 -34 59 185 m.p./℃ -220 -102 -7 114 Color pale yellow red-brown yellow-green dark red
1.Physical properties of the elemental substances: State of aggregation g g l s b.p./℃ -188 -34 59 185 m.p. /℃ -220 -102 -7 114 Color pale yellow red-brown yellow-green dark red Intermolecular force small large F2 Cl2 Br2 I2 17.2 The elemental substances

2.Chemical properties of the elemental substances ·redox properties F2 C12 Br2 2 Ee(X2X)/V:2.8891.360 1.0774 0.5345 X2 oxidizing ability:strong weak X reducing ability:weak strong Conclusion: Herein,F2:the strongest oxidant,I:the strongest reductant
F2 Cl2 Br2 I2 X2 oxidizing ability: strong weak X - reducing ability: • redox properties Herein, F2 : the strongest oxidant, I- : the strongest reductant 2. Chemical properties of the elemental substances (X 2 /X )/V: 2.889 1.360 1.0774 0.5345 - E Conclusion: weak strong
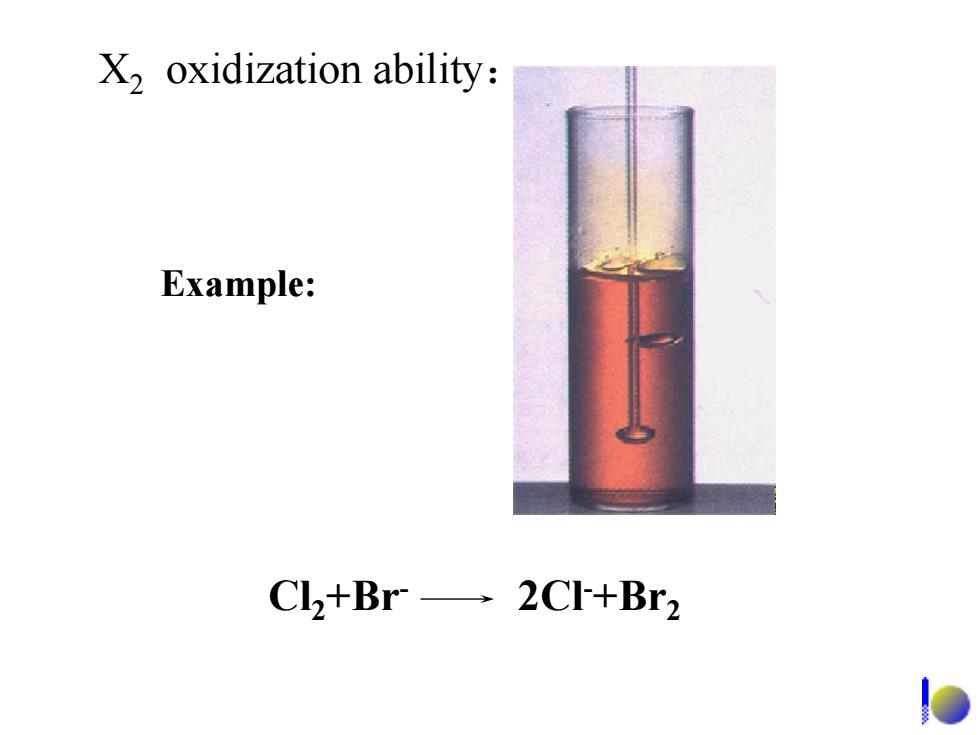
X,oxidization ability: Example: Cl+Br-2CI+Br2
Cl2+Br- 2Cl-+Br2 X2 oxidization ability: Example: