
Chapter 8 Acid-Base Equilibrium ק8.1 The Bronsted theory of acids and bases ק8.2 Ionization equilibrium of water and the pH scale X 8.3 Equilibrium in solutions of weak acids and weak bases X 8.4 Buffer solutions
Chapter 8 Acid-Base Equilibrium § 8.4 Buffer solutions § 8.3 Equilibrium in solutions of weak acids and weak bases § 8.2 Ionization equilibrium of water and the pH scale § 8.1 The Brønsted theory of acids and bases
8.5 Acid-bases indicators X 8.6 Lewis acids and bases and coordination compounds X 8.7 Complexation reaction and coordination equilibrium
§ 8.7 Complexation reaction and coordination equilibrium § 8.6 Lewis acids and bases and coordination compounds § 8.5 Acid-bases indicators

8.1 The Bronsted theory of acids and bases 8.1.1 The Bronsted acids and bases 8.1.2 Relative strengths of acids and bases
§ 8.1 The Brønsted theory of acids and bases 8.1.2 Relative strengths of acids and bases 8.1.1 The Brønsted acids and bases

Overview:(sour,bitter)Taste-→Arrhenius theory(酸碱电离理论, Ht&OHr),→Solvent theory,,Bronsted theory(质子理论),Lewis theory(电子理论),soft-hard acid/,base theory. 8.1.1 The Bronsted theory of acids and bases(酸碱质子理论) acid:a substance capable of donating a proton.(proton donor) (质子的给予体) base:a substance that can accept a proton.(proton acceptor) (质子的接受体)
acid: a substance capable of donating a proton. (proton donor) (质子的给予体) base: a substance that can accept a proton. (proton acceptor) (质子的接受体) 8.1.1 The Brønsted theory of acids and bases (酸碱质子理论) Overview: (sour, bitter) Taste Arrhenius theory(酸碱电离理论, H+&OH- ), Solvent theory, Bronsted theory(质子理论), Lewis theory(电子理论), soft-hard acid/base theory…
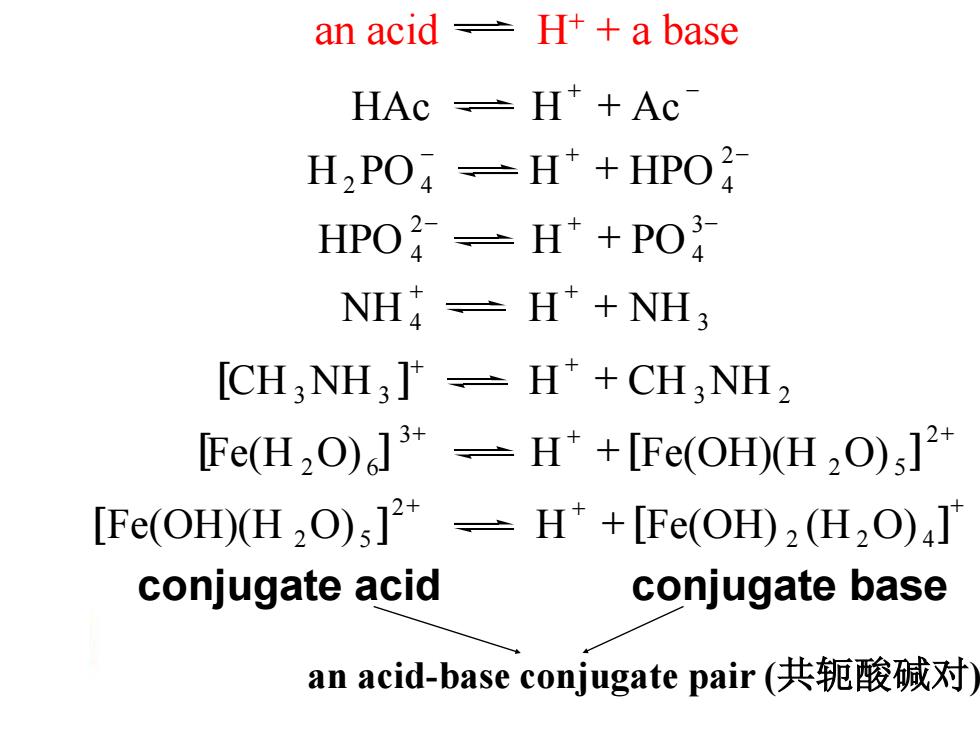
an acid 土 H++a base HAc =H*+Ac H2PO-H*+HPO2 HPO-H*+PO NH-H*+NH3 [CH:NH3]*=H*+CH3NH2 [Fe(H2O)]*-H*+[Fe(OH)(H2O);]2* [Fe(OH)(H2O);]2*-H*+[Fe(OH)2(H2O)aT conjugate acid conjugate base an acid-base conjugate pair(共轭酸碱对)
an acid H+ + a base + - HAc H + Ac - + - + 2 H2PO 4 H HPO 4 - + - + 3 4 2 HPO 4 H PO + + + NH 4 H NH 3 [ ] + + + CH 3NH 3 H CH 3NH 2 + + + + 2 2 5 3 [Fe(H 2O) 6 ] H [Fe(OH)(H O) ] + + + + 2 2 4 2 [Fe(OH)(H 2O) 5 ] H [Fe(OH) (H O) ] conjugate acid conjugate base an acid-base conjugate pair (共轭酸碱对)