
Chapter 2 Basic of thermodynamics To study chemistry using the theories and methods of thermodynamics 1)heat exchange in chemical reactions 2)directions and extent of reaction processes x 2.1 the First law of thermodynamics §2.2 Thermochemistry x 2.3 Direction of a chemical reaction
Chapter 2 Basic of thermodynamics §2.2 Thermochemistry §2.3 Direction of a chemical reaction To study chemistry using the theories and methods of thermodynamics 1) heat exchange in chemical reactions 2) directions and extent of reaction processes §2.1 the First law of thermodynamics

2.1 the First law of thermodynamics 2.1.1 Basic terms in thermodynamics 1.System and surroundings 2.State and state functions 3.Process and path 4.Volume work 5.Thermodynamic energy 2.1.2 the First law of thermodynamics 1.the Content of the First law of thermodynamics 2.Work and heat
2.1.1 Basic terms in thermodynamics 1. System and surroundings 5. Thermodynamic energy 4. Volume work 3. Process and path 2. State and state functions §2.1 the First law of thermodynamics 2.1.2 the First law of thermodynamics 1. the Content of the First law of thermodynamics 2. Work and heat

2.1 the First law of thermodynamics 2.1.1 Basic terms in thermodynamics 1.System and surroundings 2.State and state functions 3.Process and path 4.Volume work 5.Thermodynamic energy 2.1.2 the First law of thermodynamics 1.the Content of the First law of thermodynamics 2.Work and heat
2.1.1 Basic terms in thermodynamics 1. System and surroundings 5. Thermodynamic energy 4. Volume work 3. Process and path 2. State and state functions §2.1 the First law of thermodynamics 2.1.2 the First law of thermodynamics 1. the Content of the First law of thermodynamics 2. Work and heat
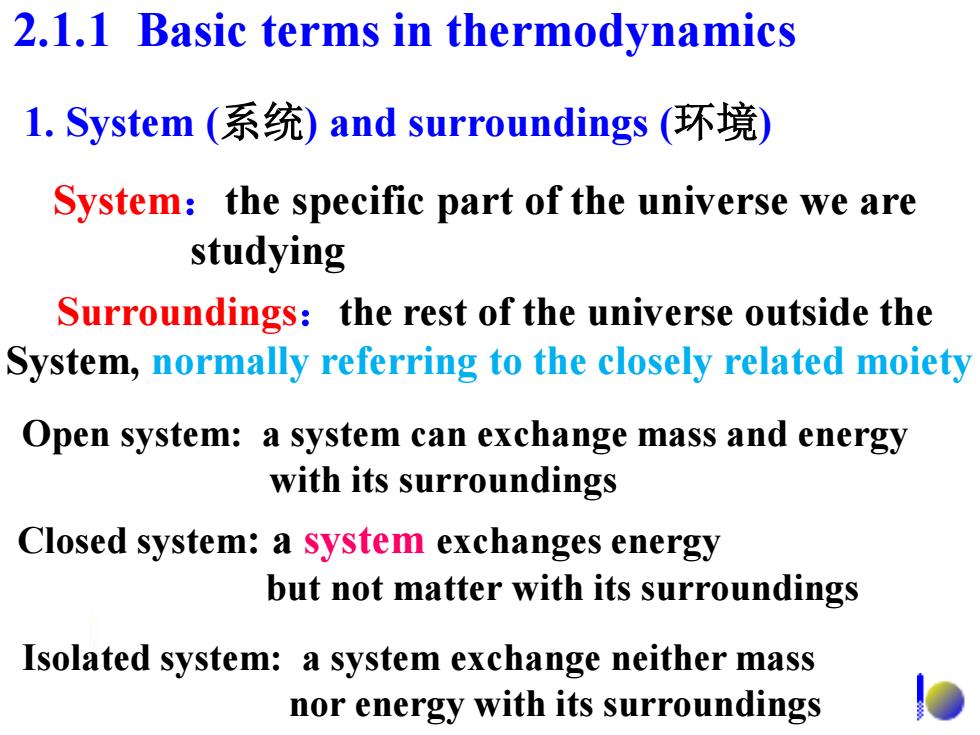
2.1.1 Basic terms in thermodynamics l.System(系统)and surroundings(环境) System:the specific part of the universe we are studying Surroundings:the rest of the universe outside the System,normally referring to the closely related moiety Open system:a system can exchange mass and energy with its surroundings Closed system:a system exchanges energy but not matter with its surroundings Isolated system:a system exchange neither mass nor energy with its surroundings
System:the specific part of the universe we are studying Surroundings:the rest of the universe outside the System, normally referring to the closely related moiety Open system: a system can exchange mass and energy with its surroundings Closed system: a system exchanges energy but not matter with its surroundings Isolated system: a system exchange neither mass nor energy with its surroundings 2.1.1 Basic terms in thermodynamics 1. System (系统) and surroundings (环境)

Three systems represented by water in a flask (a) (b) (c)
Three systems represented by water in a flask (a) (b) (c)