
Preface 1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History,revival and development of inorganic chemistry 1.4 How to study easily and efficiently inorganic chemistry
1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History, revival and development of inorganic chemistry 1.4 How to study easily and efficiently inorganic chemistry Preface
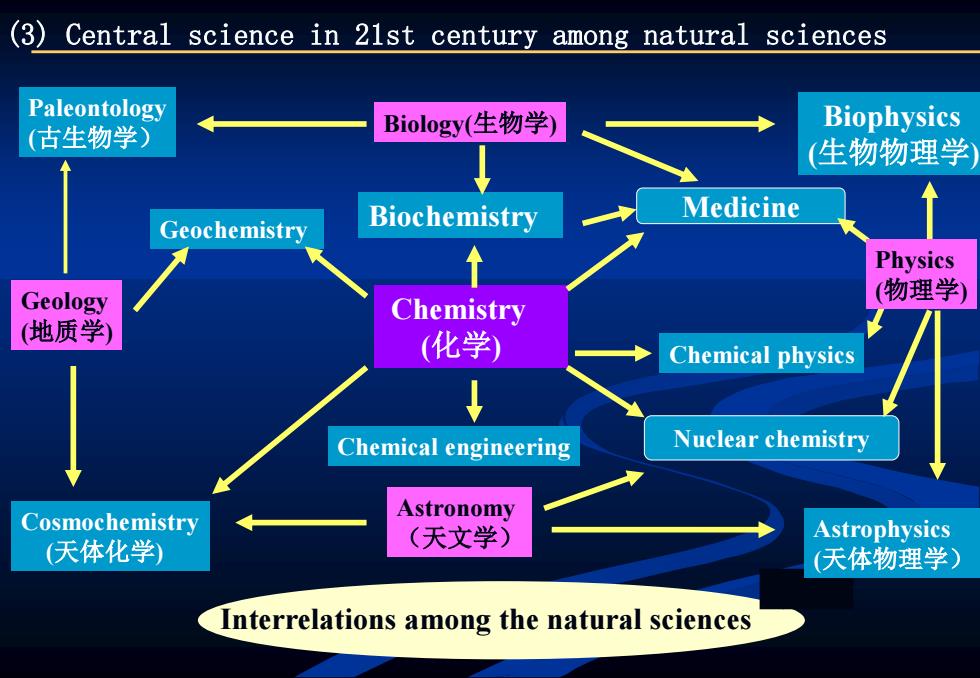
(3)Central science in 21st century among natural sciences Paleontology (古生物学) Biology(生物学) Biophysics (生物物理学 Biochemistry Medicine Geochemistry ↑ Physics Geology (物理学) Chemistry (地质学) (化学) Chemical physics Chemical engineering Nuclear chemistry Cosmochemistry Astronomy 天体化学) (天文学) Astrophysics (天体物理学) Interrelations among the natural sciences
Chemistry (化学) Biochemistry Medicine Nuclear chemistry Biophysics (生物物理学) Paleontology (古生物学) Biology(生物学) Chemical physics Chemical engineering Geology (地质学) Geochemistry Cosmochemistry (天体化学) Astronomy (天文学) Physics (物理学) Astrophysics (天体物理学) (3) Central science in 21st century among natural sciences Interrelations among the natural sciences

Preface 1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History,revival and development of inorganic chemistry 1.4 How to study easily while efficiently inorganic chemistry
1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History, revival and development of inorganic chemistry 1.4 How to study easily while efficiently inorganic chemistry Preface
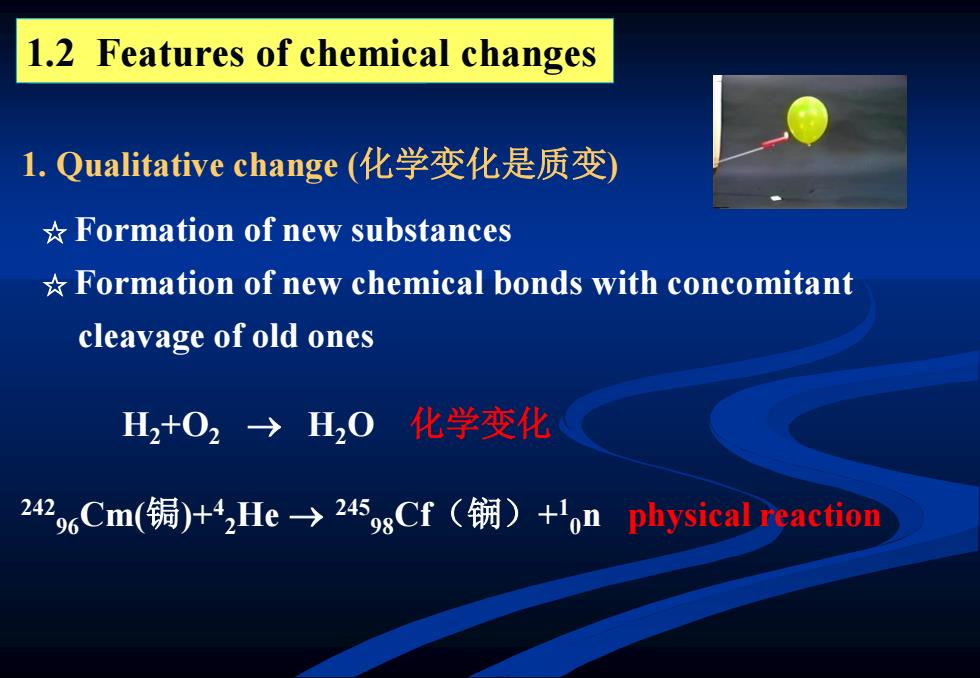
1.2 Features of chemical changes l.Qualitative change(化学变化是质变) *Formation of new substances *Formation of new chemical bonds with concomitant cleavage of old ones H2+02→H20 化学变化 242,oCm(锔)+He-→25gCf(锎)+'on physical reaction
1.2 Features of chemical changes 1. Qualitative change (化学变化是质变) 242 96Cm(锔)+4 2He 245 98Cf(锎)+ 1 0n physical reaction H2+O2 H2O 化学变化 ☆ Formation of new substances ☆ Formation of new chemical bonds with concomitant cleavage of old ones

2.Chemical reactions obey the law of mass conservation 化学反应过程中各元素的原子数和核外电子的总数没有变化 ,并且参与反应的各种物质之间有确定的计量关系 3.Chemical reactions always occur companying energy changes 化学键的断裂和形成,电子的转移伴随能量的吸收和释放,如 石油燃烧等
2. Chemical reactions obey the law of mass conservation 化学反应过程中各元素的原子数和核外电子的总数没有变化 ,并且参与反应的各种物质之间有确定的计量关系 3. Chemical reactions always occur companying energy changes 化学键的断裂和形成,电子的转移伴随能量的吸收和释放, 如 石油燃烧等