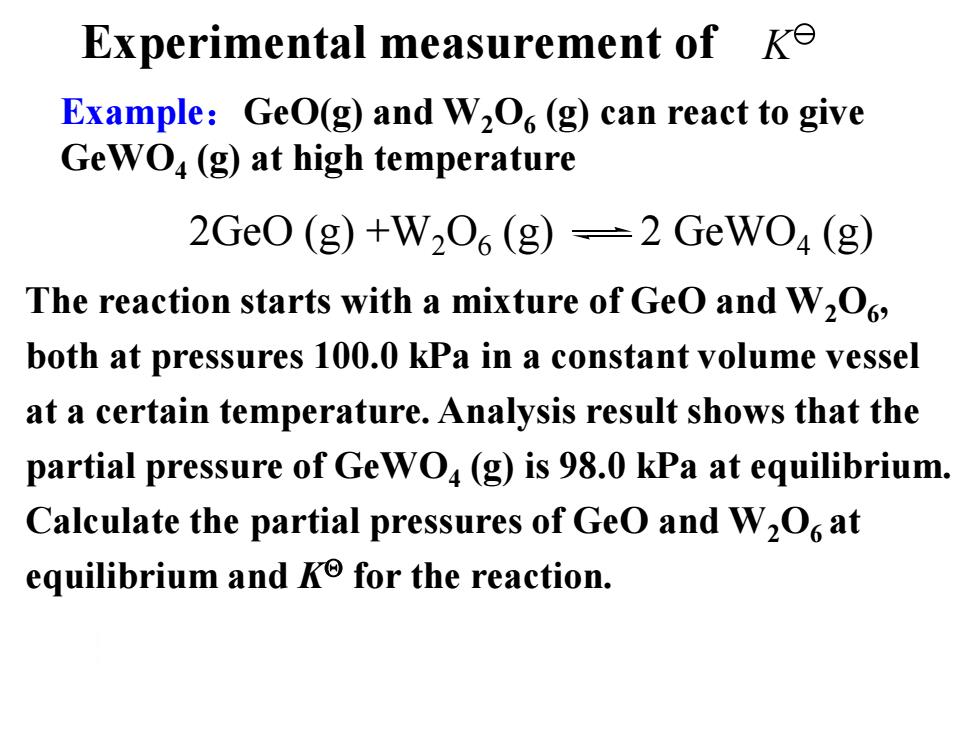
Experimental measurement of Ke Example:GeO(g)and W2O(g)can react to give GeWO4(g)at high temperature 2Ge0(g)+W2O6(g)=2GeW04(g) The reaction starts with a mixture of GeO and W2O6 both at pressures 100.0 kPa in a constant volume vessel at a certain temperature.Analysis result shows that the partial pressure of Ge WO(g)is 98.0 kPa at equilibrium. Calculate the partial pressures of GeO and W2O at equilibrium and Ke for the reaction
Example:GeO(g) and W2O6 (g) can react to give GeWO4 (g) at high temperature Experimental measurement of The reaction starts with a mixture of GeO and W2O6 , both at pressures 100.0 kPa in a constant volume vessel at a certain temperature. Analysis result shows that the partial pressure of GeWO4 (g) is 98.0 kPa at equilibrium. Calculate the partial pressures of GeO and W2O6 at equilibrium and K for the reaction. 2GeO (g) +W2O6 (g) 2 GeWO4 (g) K
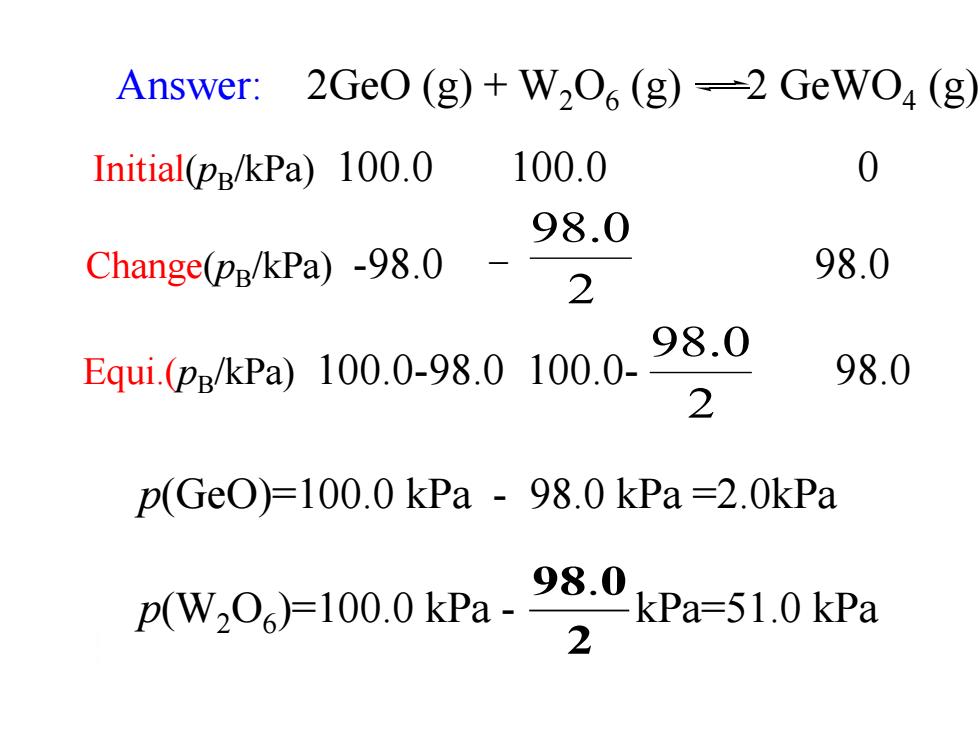
Answer: 2Ge0(g)+W206(g)=2GeW04(g, Initial(pg/kPa)100.0 100.0 0 98.0 Change(pg/kPa)-98.0 98.0 2 Equi.(ps/kPa)100.0-98.0100.0- 98.0 98.0 2 p(Ge0)=100.0kPa-98.0kPa=2.0kPa (WO)=100.0kPa-kPa=51.0kPa 2
2 98.0 p(W2O6 )=100.0 kPa - kPa=51.0 kPa p(GeO)=100.0 kPa - 98.0 kPa =2.0kPa Answer: 2GeO (g) + W2O6 (g) 2 GeWO4 (g) 2 98.0 Equi.(pB /kPa) 100.0-98.0 100.0- 98.0 Initial(pB /kPa) 100.0 100.0 0 Change(pB /kPa) -98.0 - 98.0 2 98.0
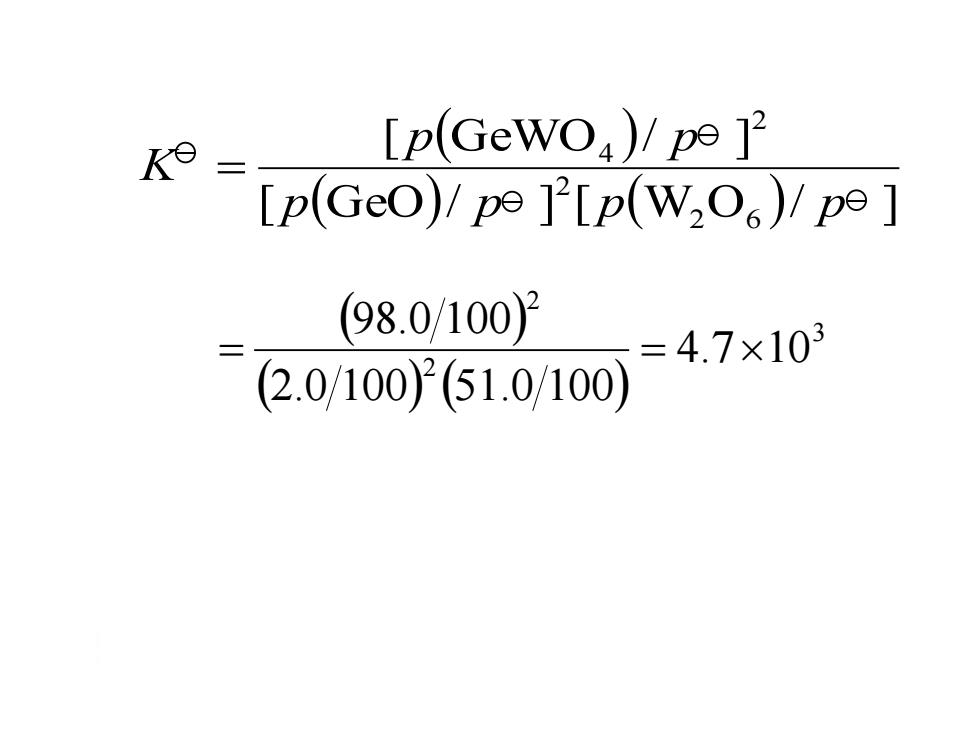
K= [p(Gewo)/pe 1 [p(GeO)/p9][p(W,O6)/p9] (98.0/1002 =4.7×103 (2.0/100)2(51.0/100)
3 2 2 4.7 10 2.0 100 51.0 100 98.0 100 [ GeO/ ] [ W O / ] [ GeWO / ] 2 6 2 2 4 p p p p p p K
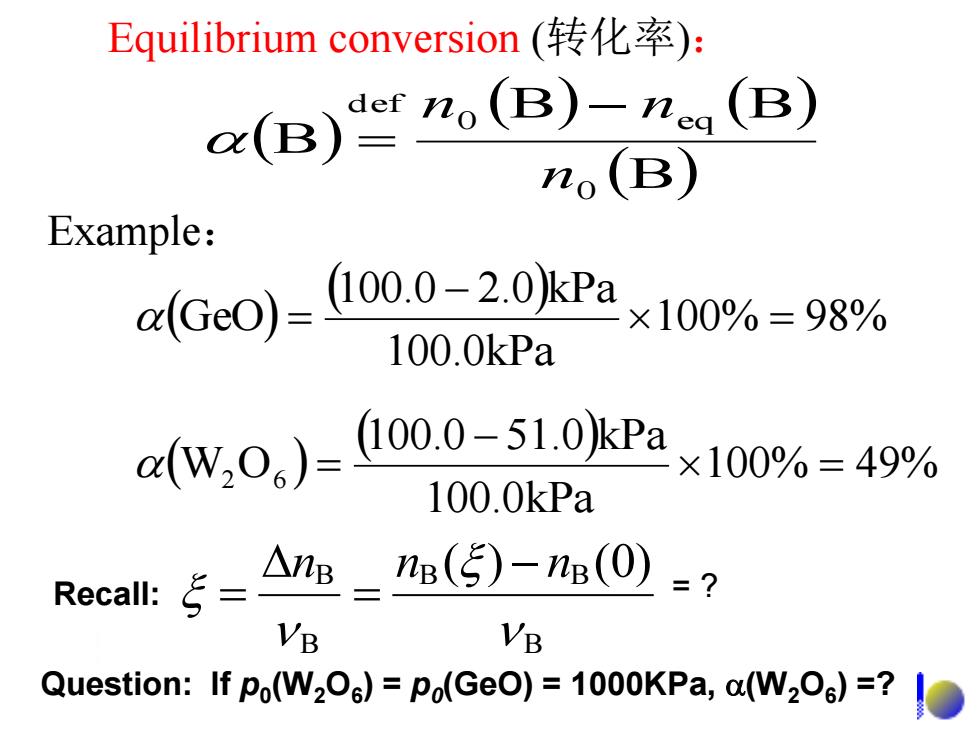
Equilibrium conversion(转化率): (B)o(B)(B) o(B) Example: rGc0)-l000-20kPax100%=98% 100.0kPa w,0.)-o00510kPa×10%=49% 100.0kPa Recall::ξ= △e-ns(5)-(0)-? VB VB Question:If po(W2O)=Po(GeO)=1000KPa,a(W2O)=?
Equilibrium conversion (转化率): B B B B 0 0 eq def n n n 100% 49% 100.0kPa 100.0 51.0 kPa W2 O6 100% 98% 100.0kPa 100.0 2.0 kPa GeO Example: Question: If p0 (W2O6 ) = p0 (GeO) = 1000KPa, (W2O6 ) =? = ? B B B B B ( ) (0) n n n Recall:
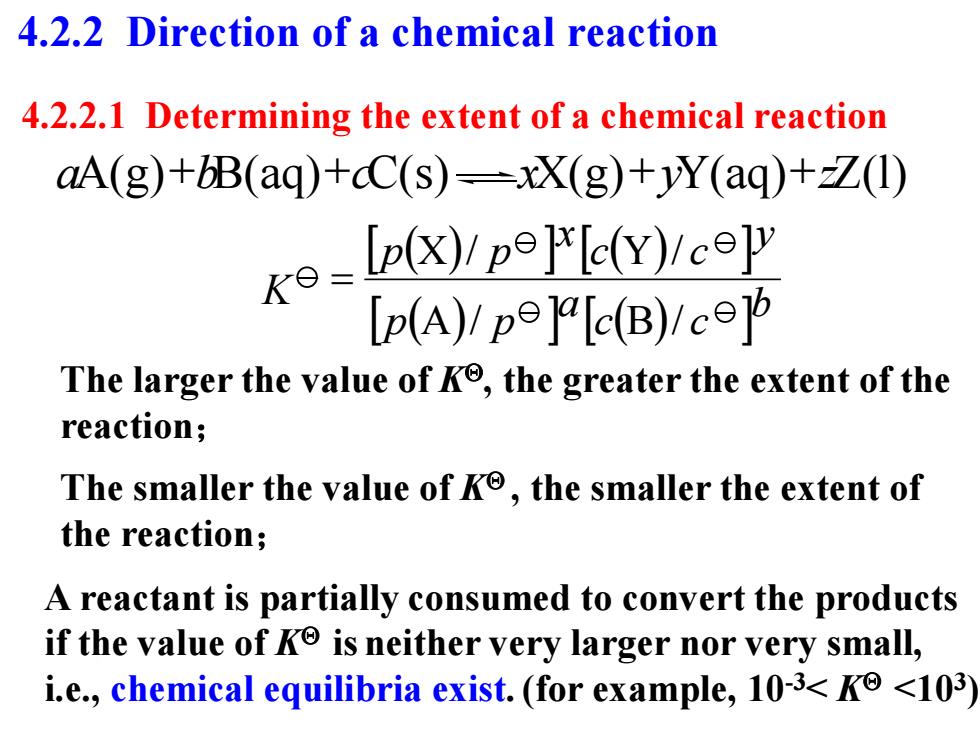
4.2.2 Direction of a chemical reaction 4.2.2.1 Determining the extent of a chemical reaction aA(g)+bB(aq)+cC(s)-xX(g)+yY(aq)+Z(1) k-lp(x)pPlc(Y)eep Ip(A)/pe]alc(B)/c The larger the value of Ko,the greater the extent of the reaction; The smaller the value of Ko,the smaller the extent of the reaction; A reactant is partially consumed to convert the products if the value of K is neither very larger nor very small, i.e.,chemical equilibria exist.(for example,10-3<Ko <103)
4.2.2.1 Determining the extent of a chemical reaction The larger the value of K, the greater the extent of the reaction; A reactant is partially consumed to convert the products if the value of K is neither very larger nor very small, i.e., chemical equilibria exist.(for example, 10-3< K <103 ) The smaller the value of K , the smaller the extent of the reaction; K b c c a p p y c c x p p A / B / X / Y / aA(g)bB(aq)cC(s) xX(g)yY(aq)zZ(l) 4.2.2 Direction of a chemical reaction