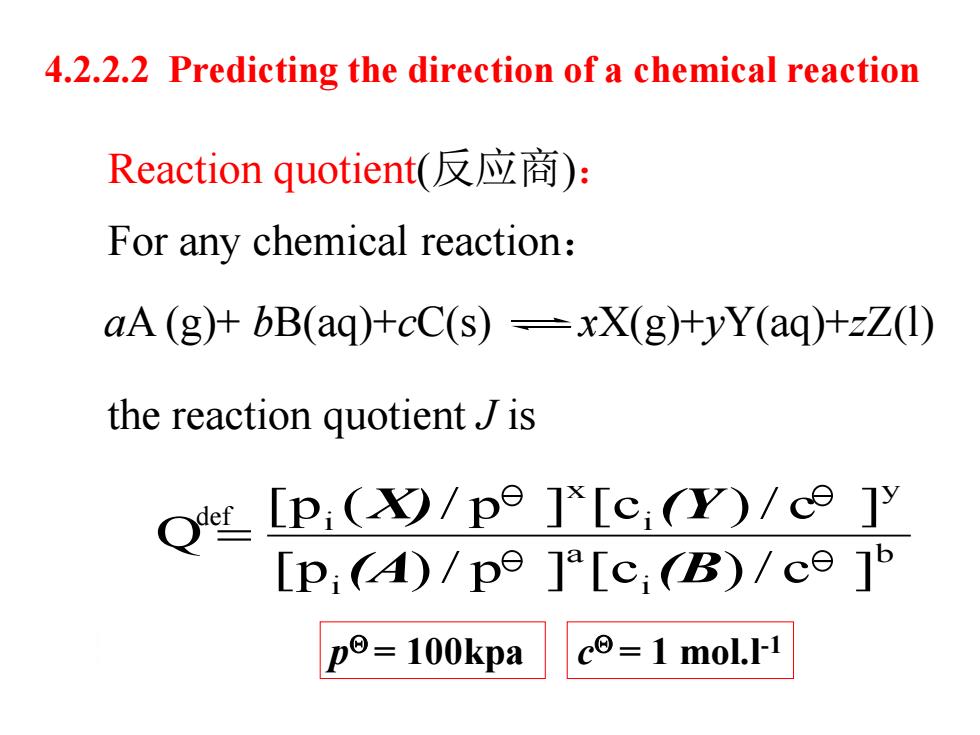
4.2.2.2 Predicting the direction of a chemical reaction Reaction quotient(反应商): For any chemical reaction: aA(g)+bB(aq)+cC(s)-xX(g)+yY(aq)+zZ(1) the reaction quotient J is OtP,(9/p9]'[c,Y)/c9]y [p;()/pe ]a[c (B)/ce]b po=100kpa c=1 mol.I-1
For any chemical reaction: 4.2.2.2 Predicting the direction of a chemical reaction the reaction quotient J is aA (g)+ bB(aq)+cC(s) xX(g)+yY(aq)+zZ(l) b i a i y i x i [p )/ p ] [c )/ c ] [p ( / p ] [c )/ c ] Q (A (B X) (Y def Reaction quotient(反应商): p = 100kpa c = 1 mol.l-1
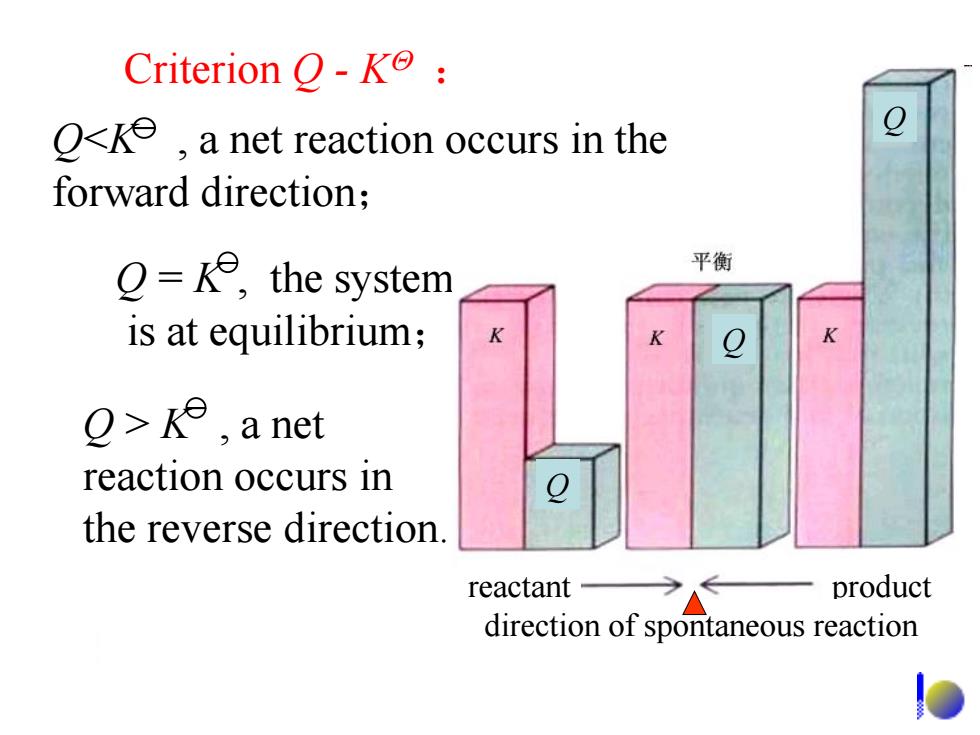
Criterion -K: O<ke,a net reaction occurs in the forward direction; =ke,the system 平衡 is at equilibrium; >k,a net reaction occurs in the reverse direction reactant product direction of spontaneous reaction
Criterion Q - K : Q = K , the system is at equilibrium; Q > K , a net reaction occurs in the reverse direction. reactant product direction of spontaneous reaction Q<K , a net reaction occurs in the forward direction; Q Q Q