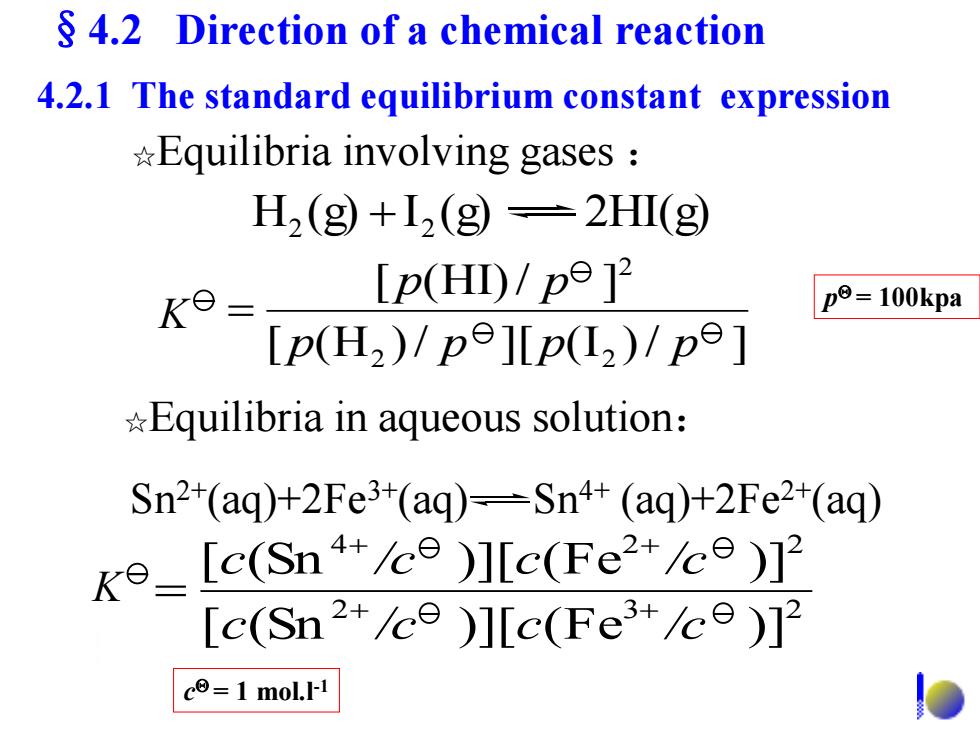
4.2 Direction of a chemical reaction 4.2.1 The standard equilibrium constant expression *Equilibria involving gases H2 (g+12(g=2HI(g Ke- [p(H)/p9]2 p=100kpa p(H2)/p9]Lp(L2)/pe] Equilibria in aqueous solution: Sn2+(aq)+2Fe3+(aq)-Sn4+(aq)+2Fe2(aq) ko_[e(Sn)]Ic(Fe2+/ce) [c(Sn2ce )]c(Fe+ce)12 c=1 mol.I-1
☆Equilibria in aqueous solution: H (g) I (g) 2HI(g) 2 2 ☆Equilibria involving gases : Sn2+(aq)+2Fe3+(aq) Sn4+ (aq)+2Fe2+(aq) [ (H )/ ][ (I )/ ] [ (HI)/ ] 2 2 2 p p p p p p K p = 100kpa 2 3 2 4 2 2 [ (Sn )][ (Fe )] [ (Sn )][ (Fe )] c /c c /c c /c c /c K c = 1 mol.l-1 4.2.1 The standard equilibrium constant expression §4.2 Direction of a chemical reaction
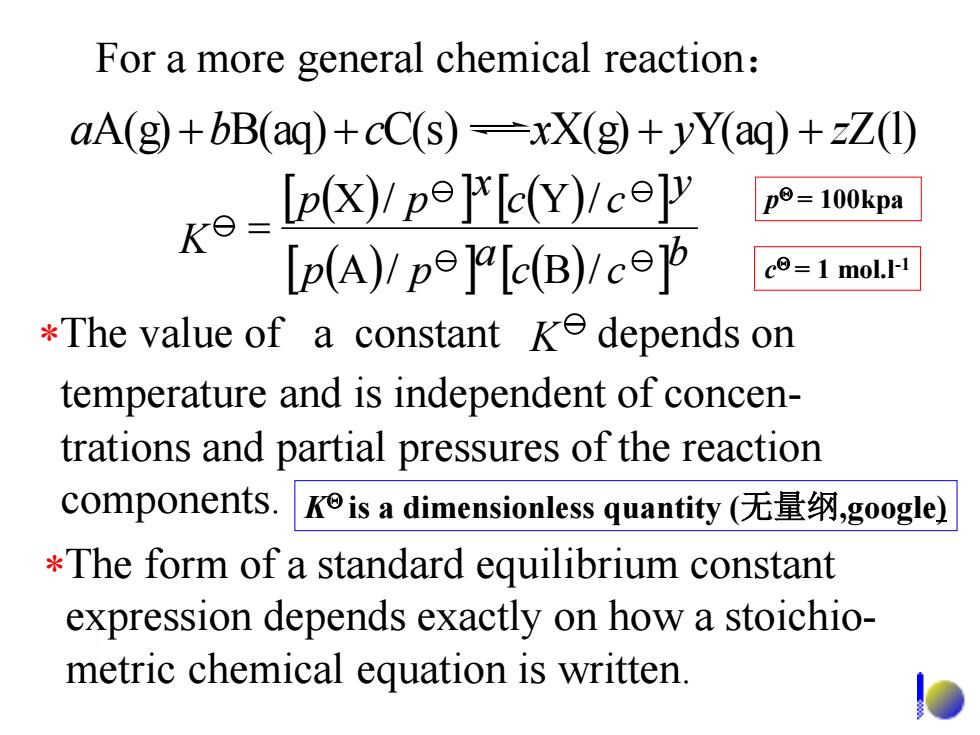
For a more general chemical reaction: aA(g)+bB(aq)+cC(s)=xX(g)+yY(aq)+zZ(1) k3=LpK/ppy/c9Ψ p=100kpa Ip(A)/pe]alc(B)Ic c=1 mol.I-1 *The value of a constant ke depends on temperature and is independent of concen- trations and partial pressures of the reaction components..Kis a dimensionless quantity(无量纲,google) *The form of a standard equilibrium constant expression depends exactly on how a stoichio- metric chemical equation is written
For a more general chemical reaction: aA(g) bB(aq) cC(s) xX(g) yY(aq) zZ(l) K b c c a p p y c c x p p A / B / X / Y / The value of a constant depends on temperature and is independent of concen- trations and partial pressures of the reaction components. K The form of a standard equilibrium constant expression depends exactly on how a stoichio- metric chemical equation is written. K is a dimensionless quantity (无量纲,google) p = 100kpa c = 1 mol.l-1
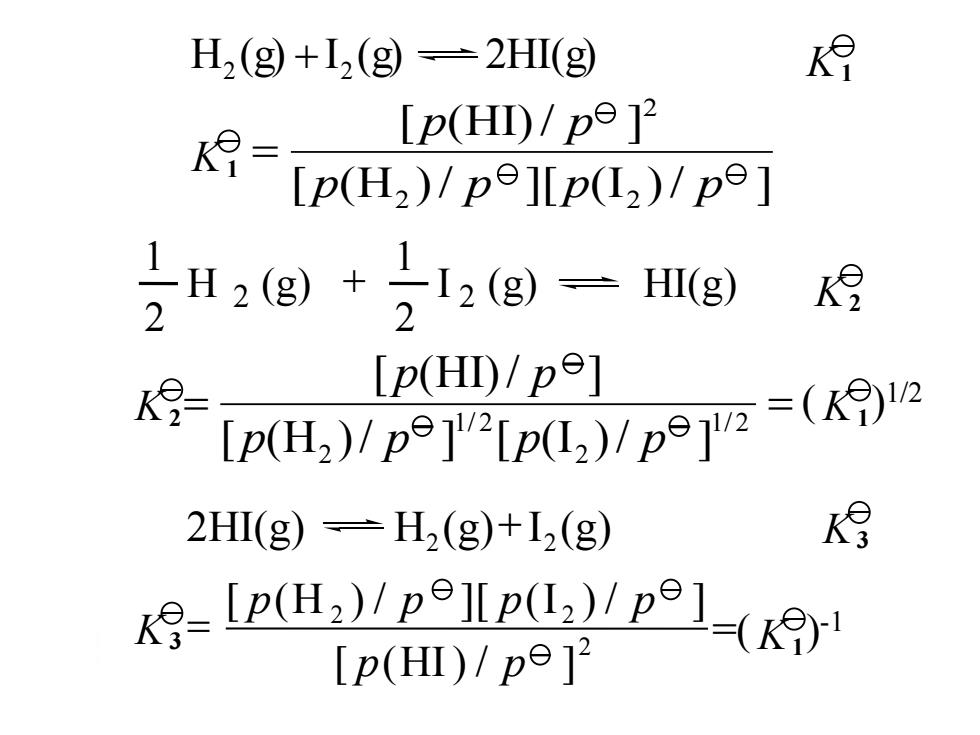
H2(g+,(g=2HI(g) [p(H)/p912 [p(H)/pe]p(I,)/pe] H2(g+号12(g)一Hg) 2 月 2 K- [P(HD)/pe] p(H)( 2HΠ(g)—H2(g)+I2(g) I(H)1-( [p(H)/pe]2
H (g) I (g) 2HI(g) 2 2 [ (H )/ ][ (I )/ ] [ (HI)/ ] 2 2 2 p p p p p p 2 I 2 (g) HI(g) 2 1 H (g) 2 1 ( )1/2 1/ 2 2 1/ 2 2 [ (H )/ ] [ (I )/ ] [ (HI)/ ] p p p p p p 2HI(g) H (g) I (g) 2 2 =( )-1 [ (H ) / ][ (I ) / ] [ (HI) / ] 2 2 2 p p p p p p K1 K1 K3 K3 K1 K2 K1 K2
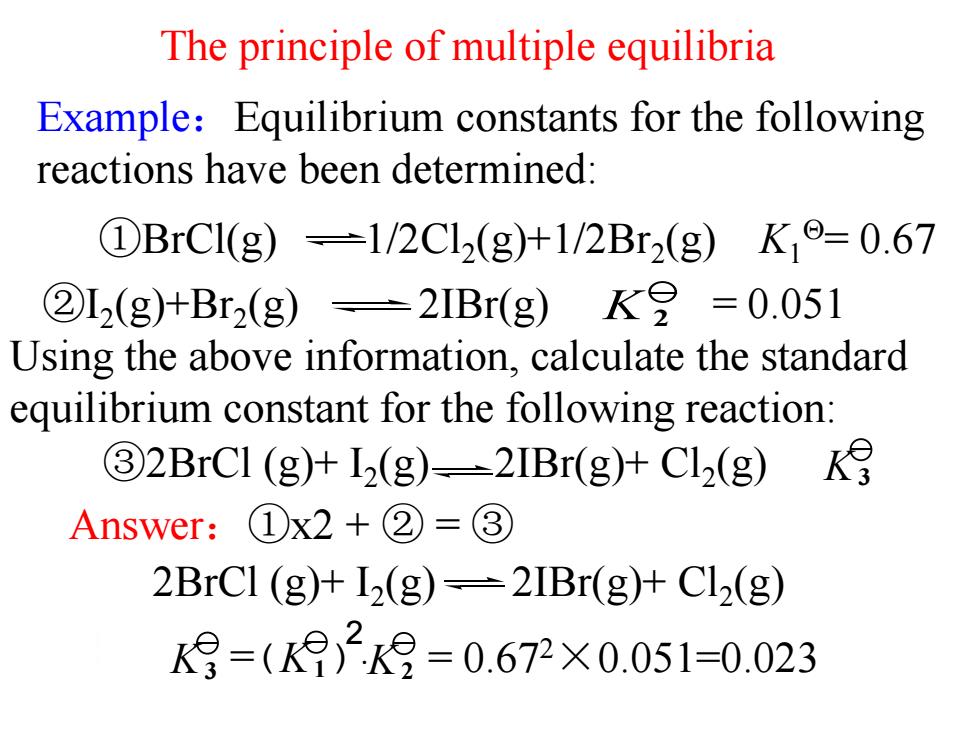
The principle of multiple equilibria Example:Equilibrium constants for the following reactions have been determined: ①BrC1(g)=1/2Cl2(g)+1/2Br2(g)K,o=0.67 ②L2(g)+Br2(g)=2IBr(g)K8=0.051 Using the above information,calculate the standard equilibrium constant for the following reaction: 32BrCI (g)+12(g)-2IBr(g)+Cl2(g) Answer:①x2+②=③ 2BrCl (g)+12(g)=2IBr(g)+Cl2(g) K月=()29=0.672X0.051=0.023
Example:Equilibrium constants for the following reactions have been determined: The principle of multiple equilibria Answer:①x2 + ② = ③ K 2 ②I2 (g)+Br2 (g) 2IBr(g) = 0.051 Using the above information, calculate the standard equilibrium constant for the following reaction: ①BrCl(g) 1/2Cl2 (g)+1/2Br2 (g) K1 = 0.67 2BrCl (g)+ I2 (g) 2IBr(g)+ Cl2 (g) ③2BrCl (g)+ I2 (g) 2IBr(g)+ Cl2 (g) K3 = · = 0.67 K3 K1 K2 2×0.051=0.023 2 ( )

The principle of multiple equilibria K月=(K2k9=0.672×0.051=0.023 Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations,the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions'reaction equilibrium constant. ------a principle of multi-equilibria 结论:如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商—多重平衡原理
The principle of multiple equilibria Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations, the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions’ reaction equilibrium constant. ------a principle of multi-equilibria 结论: 如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商多重平衡原理. = · = 0.67 K3 K 2×0.051=0.023 1 K2 2 ( )