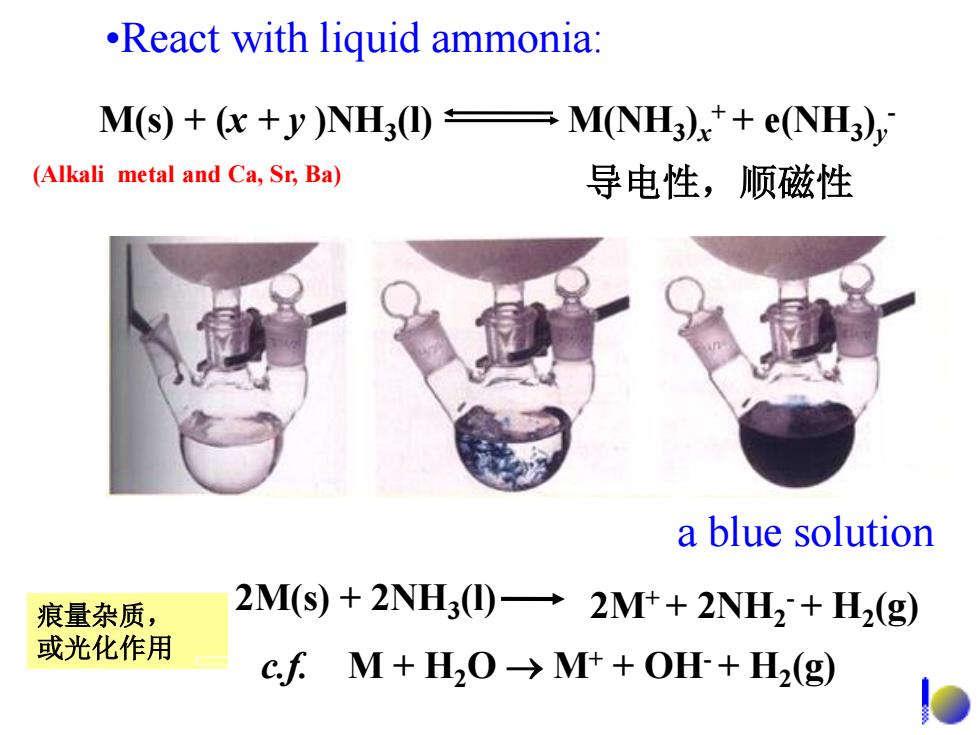
React with liquid ammonia: M(S+(c+y)NH3①= M(NH3)x+e(NH3) (Alkali metal and Ca,Sr,Ba) 导电性,顺磁性 a blue solution 痕量杂质, 2M(s)+2NH3(I)2M++2NH2+H2(g) 或光化作用 cfM+H20→Mt+OH+H2(g)
•React with liquid ammonia: a blue solution 2M(s) + 2NH3 (l) 2M+ + 2NH2 - + H2 (g) c.f. M + H2O M+ + OH- + H2 (g) M(s) + (x + y )NH3 (l) M(NH3 )x + + e(NH3 )y - 导电性,顺磁性 痕量杂质, 或光化作用 (Alkali metal and Ca, Sr, Ba)
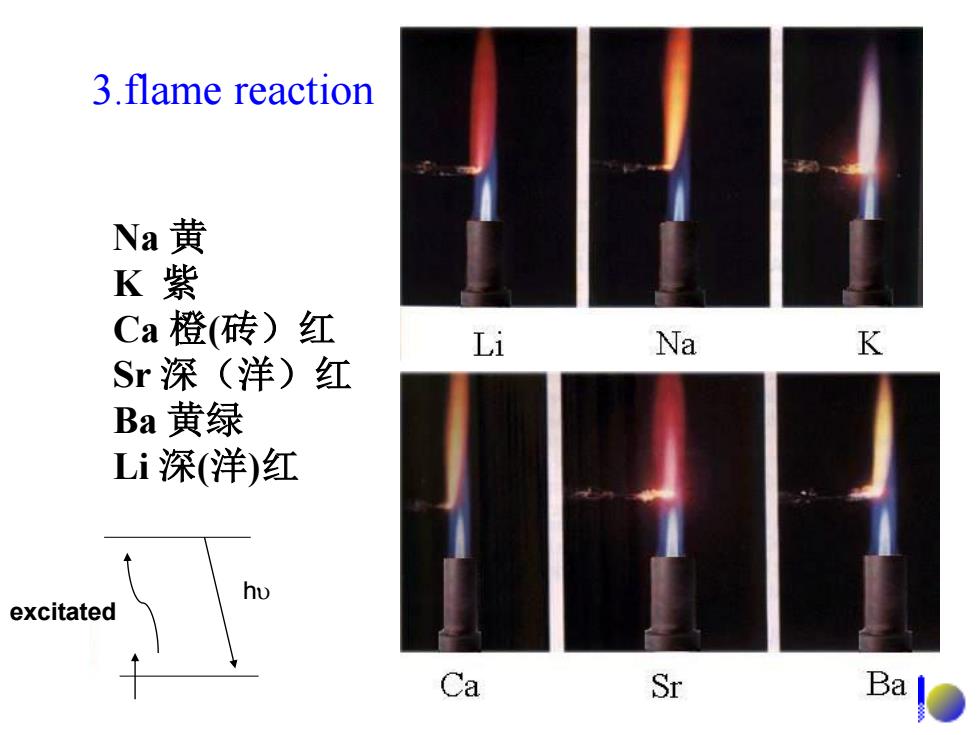
3.flame reaction Na黄 K紫 Ca橙(砖)红 Li Na K Sr深(洋)红 Ba黄绿 Li深(洋)红 excitated Ca Sr Ba
3.flame reaction Na 黄 K 紫 Ca 橙(砖)红 Sr 深(洋)红 Ba 黄绿 Li 深(洋)红 excitated h

S 12.1.2 The existence and preparation of s-block elements The s-block elements exist in minerals: Albite(钠长石):Na[AISi,Og] Potash feldspar(钾长石):K[ASi,Og] Carnallite(光卤石):KCl.MgCl2·6H2O Alunite(明矾石):K(A1O)3(SO4)2·3H2O Spodumene(锂恽石):LiAl(SiO3)2
The s-block elements exist in minerals: Na AlSi 3 O8 K AlSi 3 O8 KClMgCl 2 6H2 O K(AlO) 3 (SO4 )2 3H2 O 3 2 Spodumene (锂辉石): LiAl(SiO ) Albite (钠长石): Potash feldspar (钾长石): Carnallite (光卤石): Alunite (明矾石): § 12.1.2 The existence and preparation of s-block elements

Beyl(绿柱石):Be3A12(SiO3)6 Magnesite(菱镁矿):MgCO; Gess0(石膏):CaSO4·2H2O Marble(大理石):CaCO Fluorite(萤石):CaF2 Celestite(天青石):SrSO4 Barite(重晶石):BaSO4
Beryl (绿柱石): Magnesite (菱镁矿): Fluorite (萤石): Celestite (天青石): Marble (大理石): 3 2 3 6 Be Al (SiO ) MgCO3 CaSO4 2H2 O CaCO3 CaF2 SrSO4 BaSO4 Gesso (石膏): Barite (重晶石):

Preparation: The alkali and alkaline earth elemental substances are most conveniently obtained from molten salts by electrolysis. In addition,热还原法,金属置换法,热分解法etal (See page 465-466
Preparation: The alkali and alkaline earth elemental substances are most conveniently obtained from molten salts by electrolysis. (See page 465-466 ) In addition, 热还原法, 金属置换法, 热分解法 et al