
Chapter 22 The elements of iron and platinium families -22.1 The elements of iron family -*22.2 The elements of platinium family -22.3 The outline on the d-block elements
Chapter 22 The elements of iron and platinium families 22.1 The elements of iron family *22.2 The elements of platinium family 22.3 The outline on the d-block elements
22.1 The elements of iron family -22.1.1 The elemental substances of Fe,Co,Ni -22.1.2 The compounds of Fe,Co and Ni
22.1.1 The elemental substances of Fe, Co, Ni 22.1. 2 The compounds of Fe, Co and Ni 22.1 The elements of iron family
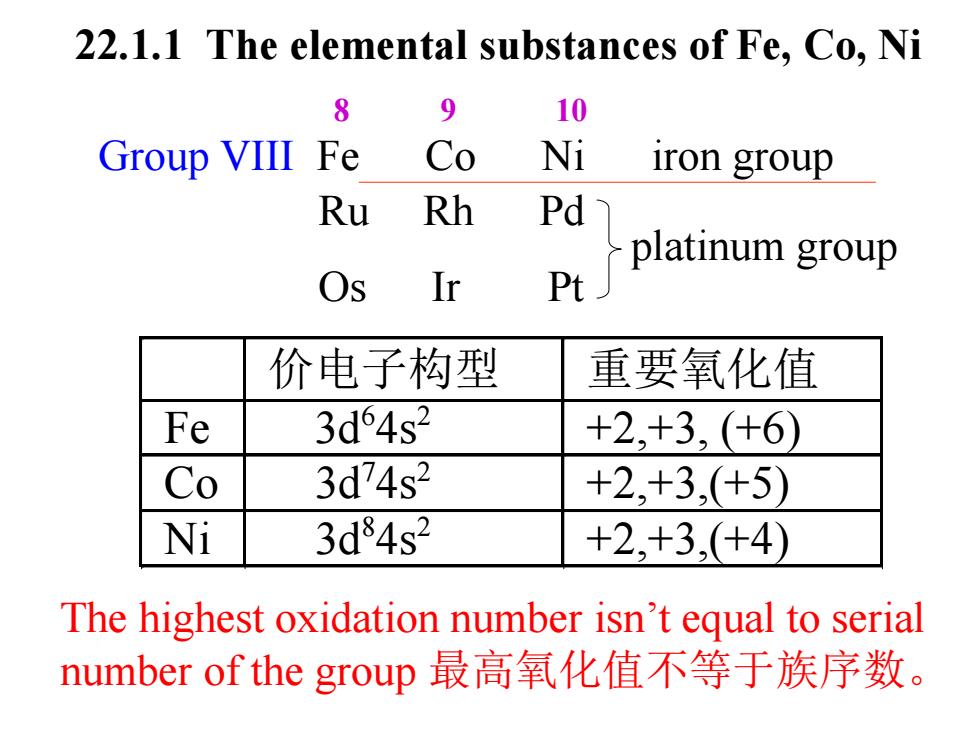
22.1.1 The elemental substances of Fe,Co,Ni 8 9 10 Group VIII Fe Co Ni iron group Ru Rh Pd Os Ir Pplatinum group 价电子构型 重要氧化值 Fe 3d64s2 +2,+3,(+6) Co 3d74s2 +2,+3,(+5) Ni 3d84s2 +2,+3,(+4) The highest oxidation number isn't equal to serial number of the group最高氧化值不等于族序数
Group VIII Fe Co Ni iron group Ru Rh Pd Os Ir Pt platinum group The highest oxidation number isn’t equal to serial number of the group 最高氧化值不等于族序数。 价电子构型 重要氧化值 Fe 3d6 4s2 +2,+3, (+6) Co 3d7 4s2 +2,+3,(+5) Ni 3d8 4s2 +2,+3,(+4) 22.1.1 The elemental substances of Fe, Co, Ni 8 9 10
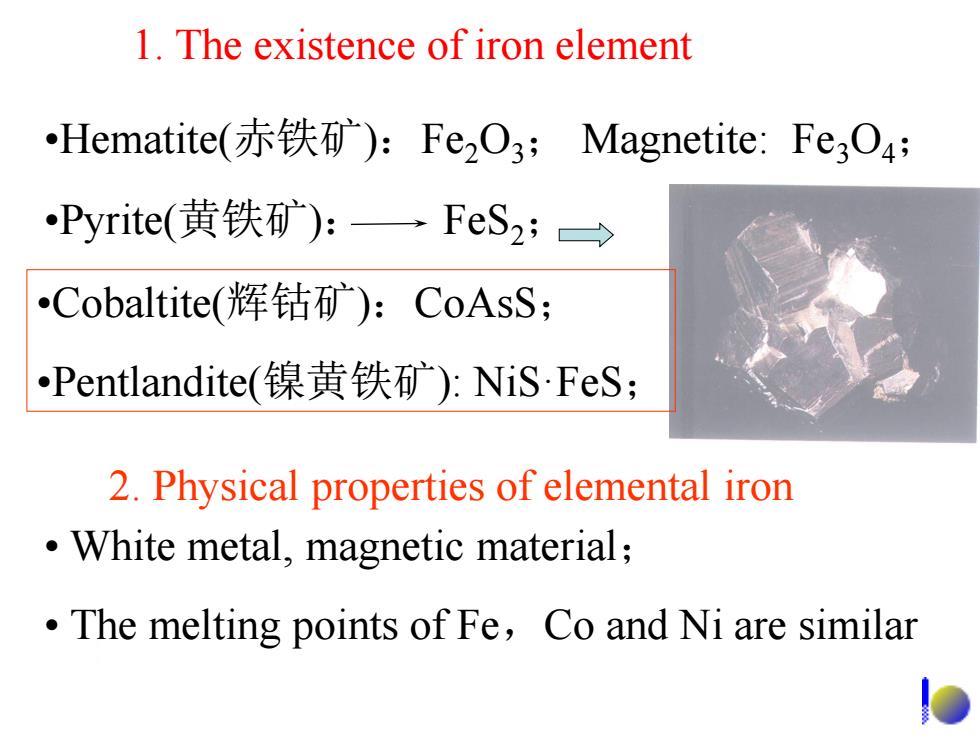
1.The existence of iron element Hematite(赤铁矿):FeO3;Magnetite:Fe3O4; Pyrite(黄铁矿):一FeS2;> Cobaltite(恽钴矿):CoAsS; Pentlandite(镍黄铁矿):NiS·FeS; 2.Physical properties of elemental iron White metal,magnetic material; The melting points of Fe,Co and Ni are similar
1. The existence of iron element •Cobaltite(辉钴矿):CoAsS; •Pentlandite(镍黄铁矿): NiS·FeS; 2. Physical properties of elemental iron • White metal, magnetic material; • The melting points of Fe,Co and Ni are similar •Hematite(赤铁矿):Fe2O3; Magnetite: Fe3O4; •Pyrite(黄铁矿): FeS2;
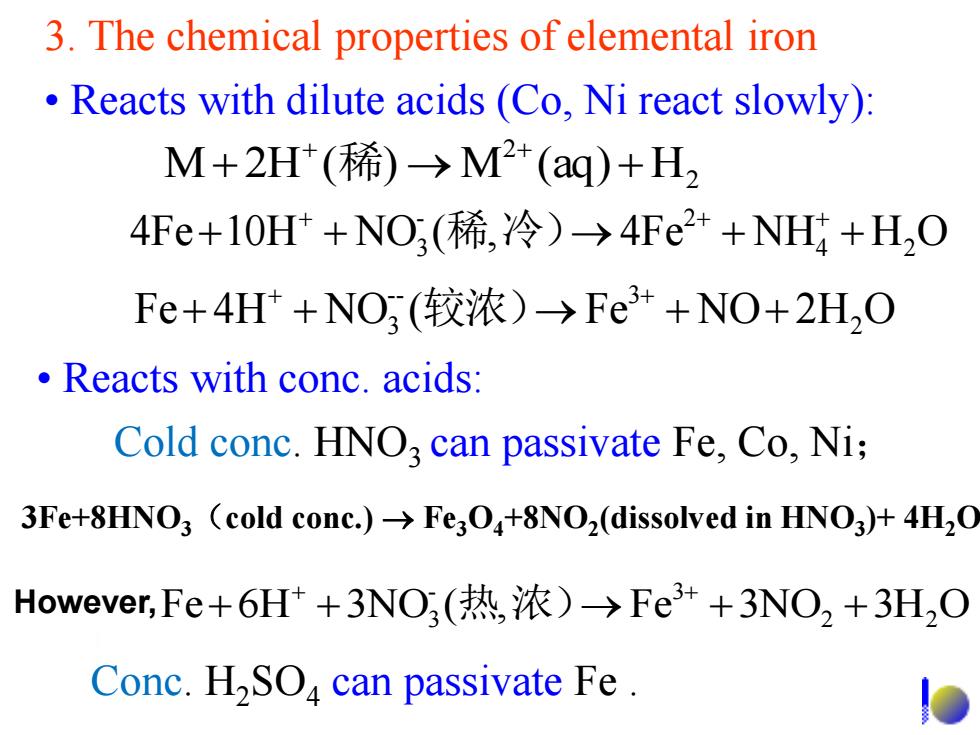
3.The chemical properties of elemental iron Reacts with dilute acids(Co,Ni react slowly): M+2H(稀)→M2+(aq)+H2 4Fe+10Ht+NO(稀,冷)→4Fe2++NH4+H,O Fe+4H+NO(较浓)→Fe3++NO+2H,O Reacts with conc.acids: Cold conc.HNO,can passivate Fe,Co,Ni; 3Fe+8HNO3 (cold conc.)>Fe3O+8NO2(dissolved in HNO3)+4H2O However,Fe+6H++3NO(热,浓)→Fe3++3NO2+3H2O Conc.H2SOa can passivate Fe
3. The chemical properties of elemental iron • Reacts with dilute acids (Co, Ni react slowly): Cold conc. HNO3 can passivate Fe, Co, Ni; 2 2 M 2H ( ) M (aq) H 稀 4Fe 10H NO ( , 4Fe NH4 H2 O - 2 3 稀 冷) Fe 4H NO ( Fe NO 2H2 O -- 3 3 较浓) • Reacts with conc. acids: Fe 6H 3NO ( , Fe 3NO2 3H2 O - 3 3 热 浓) 3Fe+8HNO3(cold conc.) Fe3O4+8NO2 (dissolved in HNO3 )+ 4H2O Conc. H2SO4 can passivate Fe . However