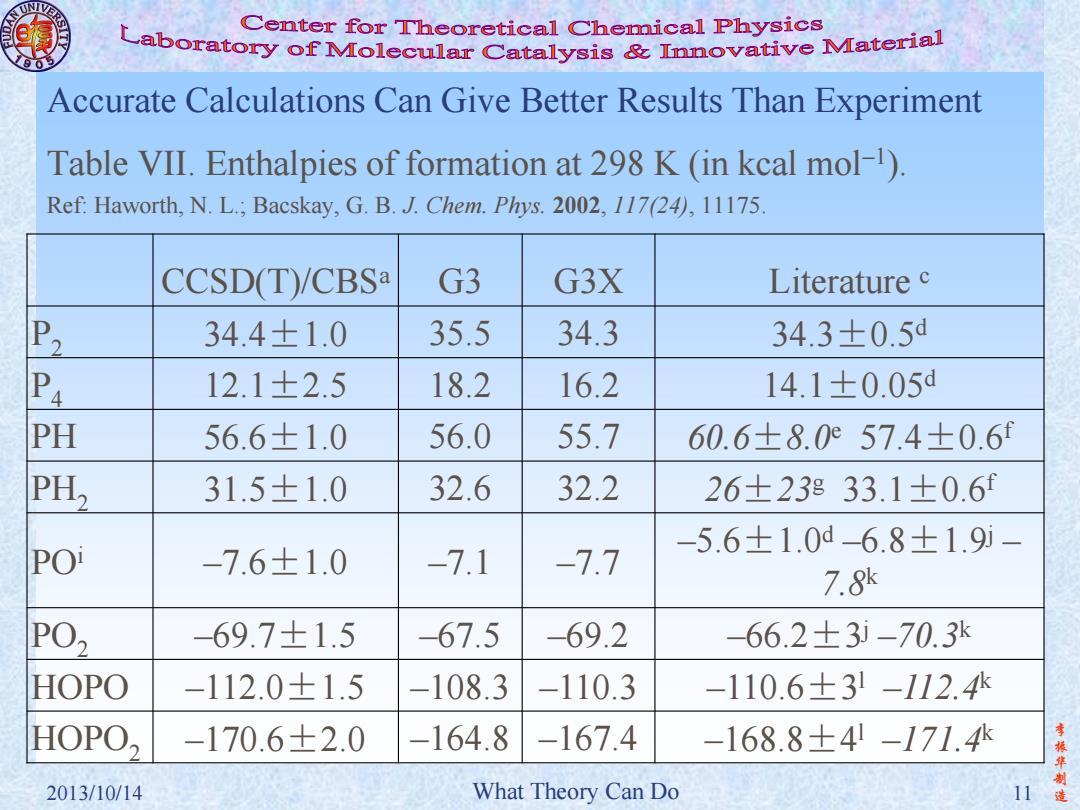
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mvative Material Accurate Calculations Can Give Better Results Than Experiment Table VII.Enthalpies of formation at 298 K (in kcal mol-1). Ref:Haworth,N.L.;Bacskay,G.B.J.Chem.Phys.2002,117(24),11175. CCSD(T)/CBSa G3 G3X Literature c P2 34.4±1.0 35.5 34.3 34.3±0.5d P4 12.1±2.5 18.2 16.2 14.1±0.05d PH 56.6±1.0 56.0 55.7 60.6±8.057.4±0.6f PH, 31.5±1.0 32.6 32.2 26±23833.1±0.6f -5.6±1.0d6.8±1.9i- POi -7.6±1.0 -7.1 -7.7 7.8 PO, -69.7±1.5 -67.5 -69.2 -66.2±3i-70.3k HOPO -112.0±1.5 -108.3 -110.3 -110.6±31-112.4 HOPO, -170.6±2.0 -164.8 -167.4 -168.8±4-171.4 李 2013/10/14 What Theory Can Do 11造
李 振 华 制 2013/10/14 What Theory Can Do 11 造 Accurate Calculations Can Give Better Results Than Experiment CCSD(T)/CBSa G3 G3X Literature c P2 34.4±1.0 35.5 34.3 34.3±0.5d P4 12.1±2.5 18.2 16.2 14.1±0.05d PH 56.6±1.0 56.0 55.7 60.6±8.0e 57.4±0.6f PH2 31.5±1.0 32.6 32.2 26±23g 33.1±0.6f POi –7.6±1.0 –7.1 –7.7 –5.6±1.0d –6.8±1.9j – 7.8k PO2 –69.7±1.5 –67.5 –69.2 –66.2±3 j –70.3k HOPO –112.0±1.5 –108.3 –110.3 –110.6±3 l –112.4k HOPO2 –170.6±2.0 –164.8 –167.4 –168.8±4 l –171.4k Table VII. Enthalpies of formation at 298 K (in kcal mol–1 ). Ref: Haworth, N. L.; Bacskay, G. B. J. Chem. Phys. 2002, 117(24), 11175
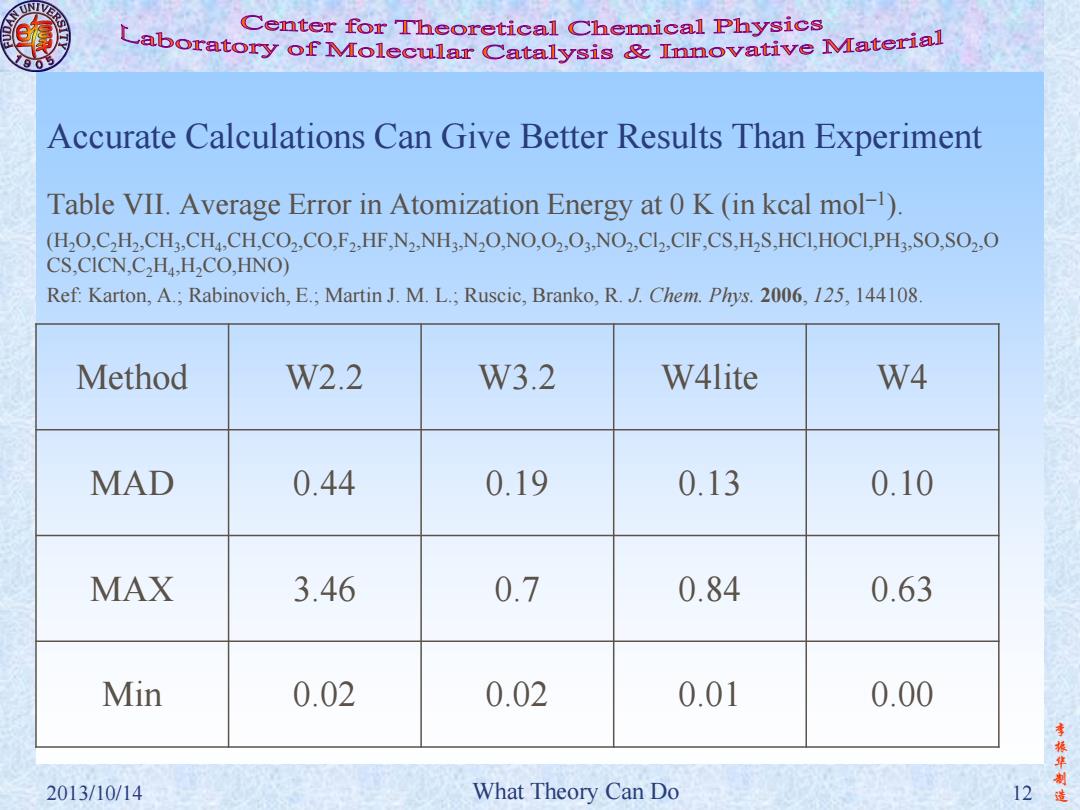
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis Innovative Material Accurate Calculations Can Give Better Results Than Experiment Table VII.Average Error in Atomization Energy at 0 K(in kcal mol-1) (HO.C,H2,CH3,CH.,CH.CO2,CO.F2,HF.N2,NH3.N2O.NO.O2,O..NO2,Cl2,ClF,CS.H2S,HCI,HOCI.PH3,SO.SO2,O CS,CICN,C,H4,H2CO,HNO) Ref:Karton,A.;Rabinovich,E.;Martin J.M.L.;Ruscic,Branko,R.J.Chem.Phys.2006,125,144108 Method W2.2 W3.2 W4lite W4 MAD 0.44 0.19 0.13 0.10 MAX 3.46 0.7 0.84 0.63 Min 0.02 0.02 0.01 0.00 振华制 2013/10/14 What Theory Can Do 12
李 振 华 制 2013/10/14 What Theory Can Do 12 造 Accurate Calculations Can Give Better Results Than Experiment Table VII. Average Error in Atomization Energy at 0 K (in kcal mol–1 ). (H2O,C2H2 ,CH3 ,CH4 ,CH,CO2 ,CO,F2 ,HF,N2 ,NH3 ,N2O,NO,O2 ,O3 ,NO2 ,Cl2 ,ClF,CS,H2S,HCl,HOCl,PH3 ,SO,SO2 ,O CS,ClCN,C2H4 ,H2CO,HNO) Ref: Karton, A.; Rabinovich, E.; Martin J. M. L.; Ruscic, Branko, R. J. Chem. Phys. 2006, 125, 144108. Method W2.2 W3.2 W4lite W4 MAD 0.44 0.19 0.13 0.10 MAX 3.46 0.7 0.84 0.63 Min 0.02 0.02 0.01 0.00

Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mvative Material Accurate Calculations Can Give Better Results Than Experiment Table I.Recommended Gas-Phase Heats of Formation at 0 K(in kcal mol-1). Ref:Karton,A.:Martin J.M.L.J.Phys.Chem.A 2007,111(26),5936. element JANAF CODATA OPW Theo.Recom. Be 76.42±1.20 75.8±0.8 76.4±0.6 B 132.6 133.82±1.20 136.2±0.2 135.1±0.2 Al 78.23 78.30±0.96 80.2±0.4 Si 106.5±1.9 108.1±0.5 107.15±0.2 振华制 2013/10/14 What Theory Can Do 13造
李 振 华 制 2013/10/14 What Theory Can Do 13 造 Accurate Calculations Can Give Better Results Than Experiment Table I. Recommended Gas-Phase Heats of Formation at 0 K (in kcal mol–1 ). Ref: Karton, A.; Martin J. M. L. J. Phys. Chem. A 2007, 111(26), 5936. element JANAF CODATA OPW9 Theo. Recom. Be 76.42±1.20 75.8±0.8 76.4±0.6 B 132.6 133.82±1.20 136.2±0.2 135.1±0.2 Al 78.23 78.30±0.96 80.2±0.4 Si 106.5±1.9 108.1±0.5 107.15±0.2
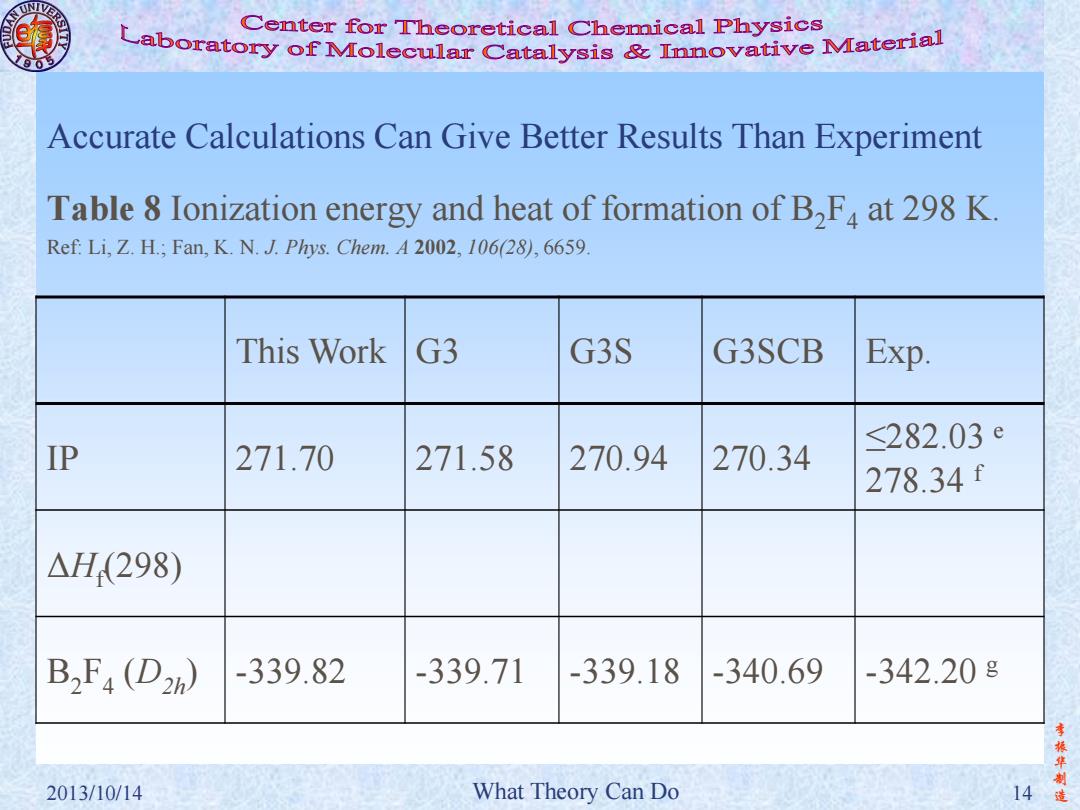
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mvative Material Accurate Calculations Can Give Better Results Than Experiment Table 8 Ionization energy and heat of formation of B,F at 298 K Ref:Li,Z.H.;Fan,K.N.J.Phys.Chem.A 2002,106(28),6659. This Work G3 G3S G3SCB Exp. ≤282.03e IP 271.70 271.58 270.94 270.34 278.34f △H(298) B2F4(D2) -339.82 -339.71 -339.18-340.69 -342.208 李 华制 2013/10/14 What Theory Can Do 14造
李 振 华 制 2013/10/14 What Theory Can Do 14 造 Accurate Calculations Can Give Better Results Than Experiment Table 8 Ionization energy and heat of formation of B2F4 at 298 K. Ref: Li, Z. H.; Fan, K. N. J. Phys. Chem. A 2002, 106(28), 6659. This Work G3 G3S G3SCB Exp. IP 271.70 271.58 270.94 270.34 ≤282.03 e 278.34 f ΔHf (298) B2 F4 (D2h) -339.82 -339.71 -339.18 -340.69 -342.20 g
Center for Theoretical Chemical Physics -aboratory of Molecular Catalysis Innovative Materia Weakness Results sensitive to Basis set, Method Very Expensive For Reliable Predictions Wrong conclusion from inaccurate calculations DFT:Good in geometry,frequency,long way to reliable quantitative predictions 李振华制 2013/10/14 What Theory Can Do 15
李 振 华 制 2013/10/14 What Theory Can Do 15 造 Weakness Results sensitive to Basis set, Method Very Expensive For Reliable Predictions Wrong conclusion from inaccurate calculations DFT: Good in geometry, frequency, long way to reliable quantitative predictions