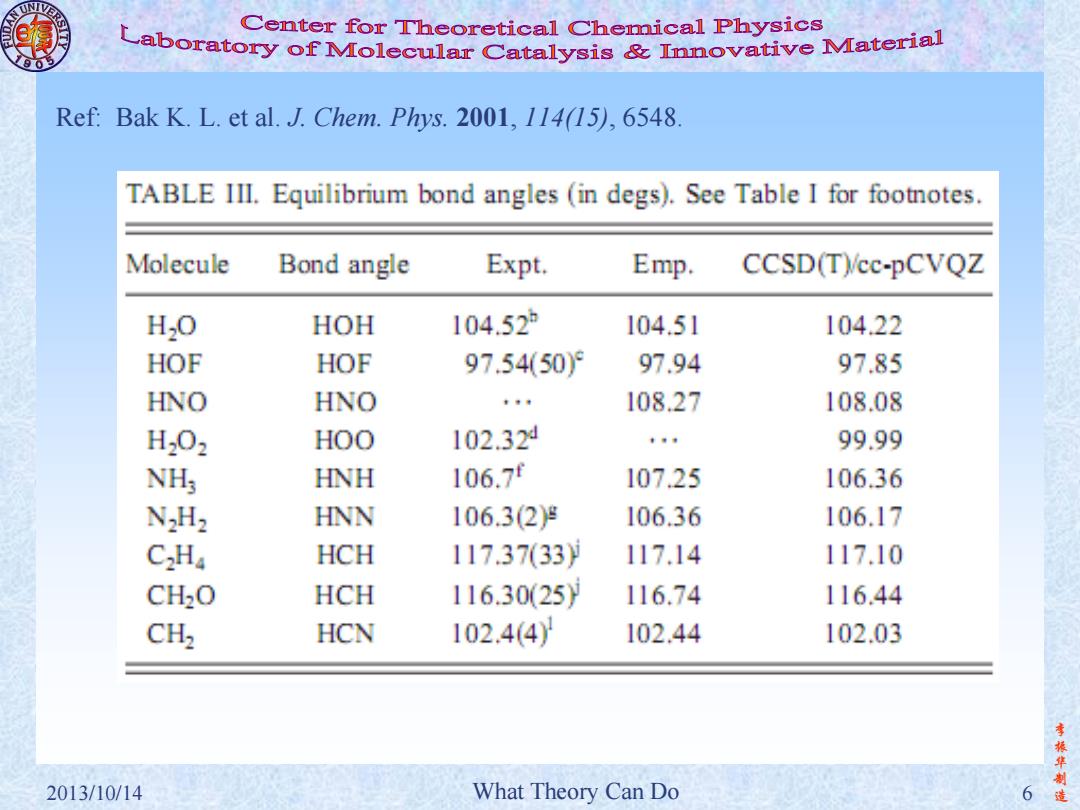
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis Innovative Material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548 TABLE III.Equilibrium bond angles (in degs).See Table I for footnotes. Molecule Bond angle Expt. Emp. CCSD(Tcc-pCVQZ H2O HOH 104.52 104.51 104.22 HOF HOF 97.54(50)9 97.94 97.85 HNO HNO t 108.27 108.08 H202 HOO 102.32 ,9 99.99 NHy HNH 106.7 107.25 106.36 N2H2 HNN 106.3(2g 106.36 106.17 C2Ha HCH 117.3733) 117.14 117.10 CH2O HCH 116.3025) 116.74 116.44 CH2 HCN 102.4(4 102.44 102.03 李 振华制 2013/10/14 What Theory Can Do 6
李 振 华 制 2013/10/14 What Theory Can Do 6 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548
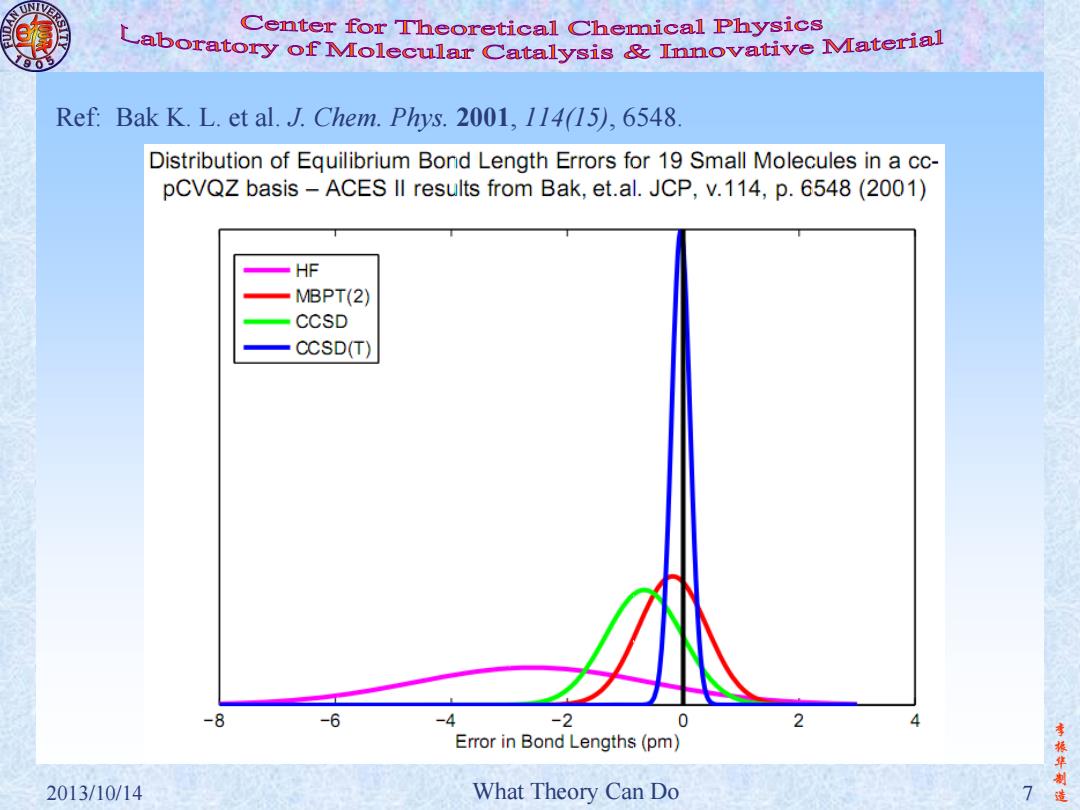
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mnovative Material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548. Distribution of Equilibrium Bond Length Errors for 19 Small Molecules in a cc- pCVQZ basis-ACES lI results from Bak,et.al.JCP,v.114,p.6548(2001) HF 一MBPT(2) CCSD 一CCSD() -8 -6 -4 -2 0 Error in Bond Lengths (pm) 李振华制 2013/10/14 What Theory Can Do
李 振 华 制 2013/10/14 What Theory Can Do 7 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548
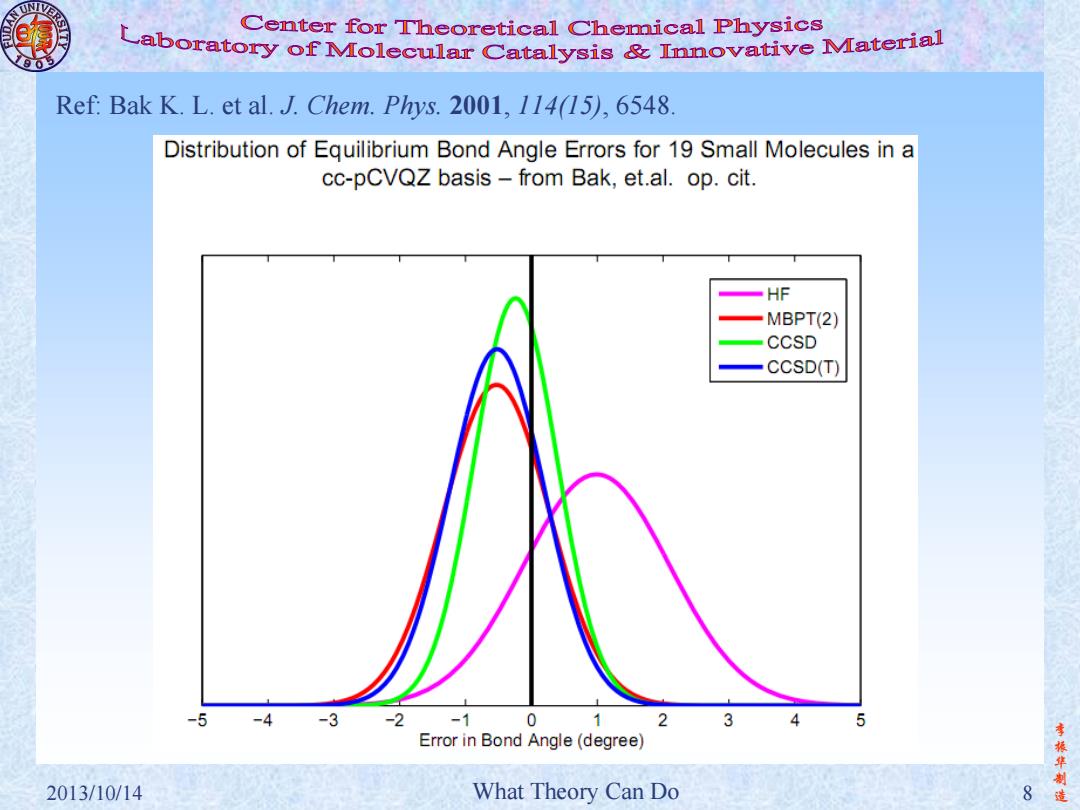
Center for Theoretical Chemical Physics Laboratory of molecular Catalysis Innovative material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548. Distribution of Equilibrium Bond Angle Errors for 19 Small Molecules in a cc-pCVQZ basis from Bak,et.al.op.cit. -HF MBPT(2) CCSD CCSD(T) -5-4-3-2 -1 0 2 1 3 4 Error in Bond Angle(degree) 振华制 2013/10/14 What Theory Can Do 8
李 振 华 制 2013/10/14 What Theory Can Do 8 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548

Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis Iovative Material Ref:Abrams,M.L.PhD Thesis,General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry,2005 Table 6:Spectroscopic constants of X 3>-NH. Method Energy Te We WeTe Be De Qe De FCI/DZP -25.208881 1.2491 2339 47.6 11.700 0.12e-02 0.399 3.48 FCI/6-31G*(⑤d) -25.206493 1.2346 2392 52.2 11.977 0.12e-02 0.429 3.51 FCI/6-31G** -25.207157 1.2344 2388 52.0 11.980 0.12e-02 0.431 3.51 FCI/cc-pVDZ -25.215324 1.2559 2340 48.8 11.574 0.11e-02 0.396 3.44 FCI/cc-pVDZ(6d) -25.216182 1.2553 2343 49.0 11.584 0.11e-02 0.398 3.44 FCI/WMR-ANO -25.214743 1.2675 2309 49.5 11.364 0.11e-02 0.388 3.47 FCI/6-31G**(opt) -25.211093 1.2446 2376 52.5 11.786 0.12e-02 0.415 3.49 FCI/DZP-NO -25.229338 1.2366 2354 50.9 11.940 0.12e-02 0.457 3.57 FCI/DZP-NO(5Z) -25.229704 1.2362 2350 51.8 11.948 0.12e-02 0.446 3.57 Experiment 1.2324 2367 49.4 12.021 0.12e-02 0.412 3.57 Energies in a.u.,re in A,De in eV,and all other quantities in cm-1. 振华制 2013/10/14 What Theory Can Do 造
李 振 华 制 2013/10/14 What Theory Can Do 9 造 Ref: Abrams, M. L. PhD Thesis, General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry, 2005
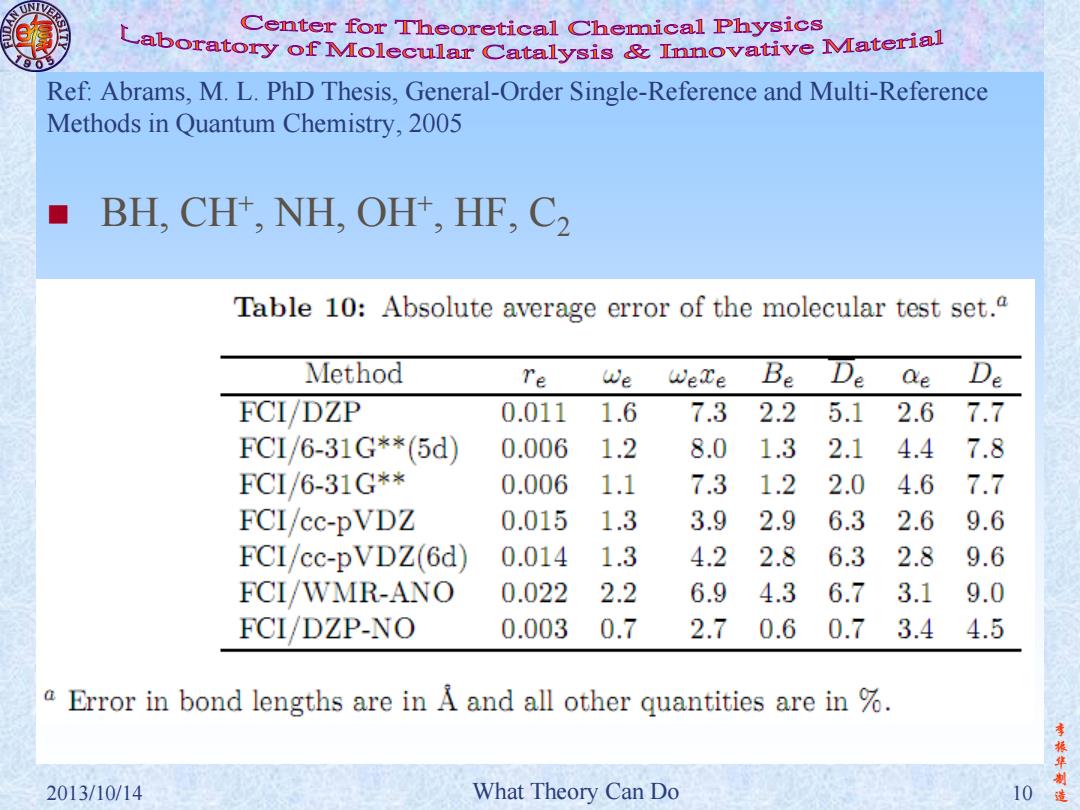
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mnovative Material Ref:Abrams,M.L.PhD Thesis,General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry,2005 BH,CH+,NH,OH+,HF,C2 Table 10:Absolute average error of the molecular test set.a Method Te We WeTe Be De Qe De FCI/DZP 0.011 1.6 7.3 2.2 5.1 2.67.7 FCI/6-31G**(5d) 0.006 1.2 8.0 1.3 2.1 4.47.8 FCL/6-31G** 0.006 1.1 7.3 1.2 2.0 4.6 7.7 FCI/cc-pVDZ 0.015 1.3 3.9 2.9 6.3 2.6 9.6 FCI/cc-pVDZ(6d) 0.014 1.3 4.2 2.8 6.3 2.8 9.6 FCI/WMR-ANO 0.022 2.2 6.9 4.3 6.7 3.1 9.0 FCI/DZP-NO 0.003 0.7 2.7 0.6 0.7 3.4 4.5 a Error in bond lengths are in A and all other quantities are in % 李 振华 2013/10/14 What Theory Can Do 10
李 振 华 制 2013/10/14 What Theory Can Do 10 造 Ref: Abrams, M. L. PhD Thesis, General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry, 2005 BH, CH+ , NH, OH+ , HF, C2