
Work (W):Work is an energy transfer into or out of a system except heat. Work is not a state function Prescript:a system does work on its surroundings, W<0 (lose work) the surroundings does work on a system W>0 (get work) Work can be of two types: volume work non-volume work (Electrical,surface work etc)
Work is an energy transfer into or out of a system except heat. a system does work on its surroundings, W<0(lose work) the surroundings does work on a system , W>0(get work) Work (W ): Prescript: non-volume work ( Electrical, surface work etc) Work is not a state function Work can be of two types: volume work
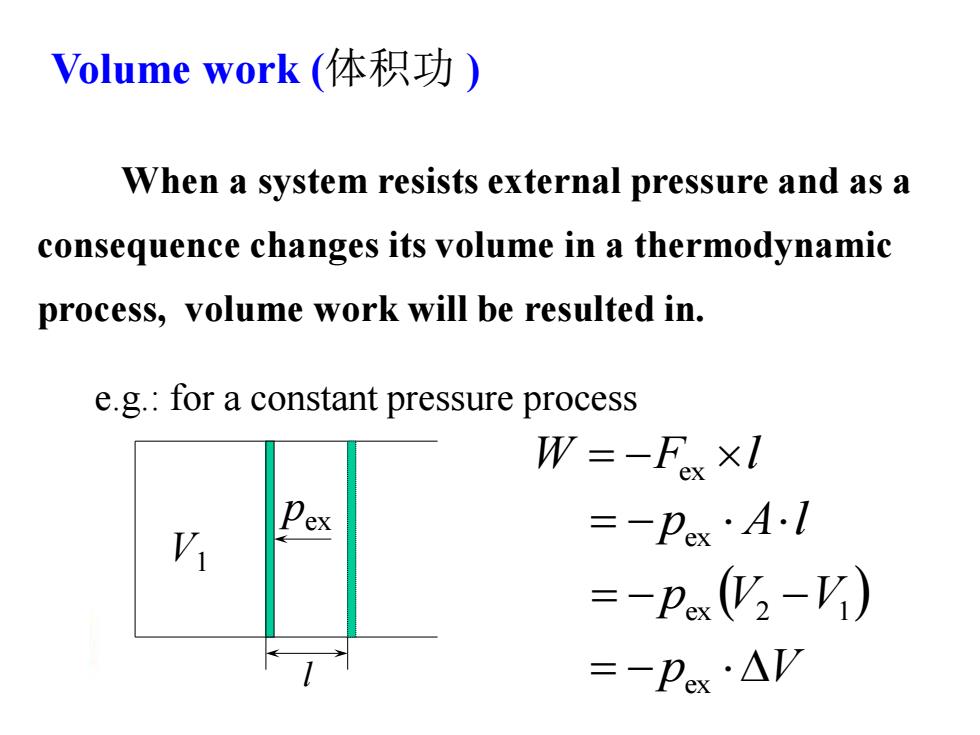
Volume work(体积功) When a system resists external pressure and as a consequence changes its volume in a thermodynamic process,volume work will be resulted in. e.g.:for a constant pressure process W=-Fx×I V =-Pex·AI =-p(y2-V) =-Pex·AV
Volume work (体积功 ) When a system resists external pressure and as a consequence changes its volume in a thermodynamic process, volume work will be resulted in. pex V1 l W F l ex p Al ex pex V2 V1 pex V e.g.: for a constant pressure process
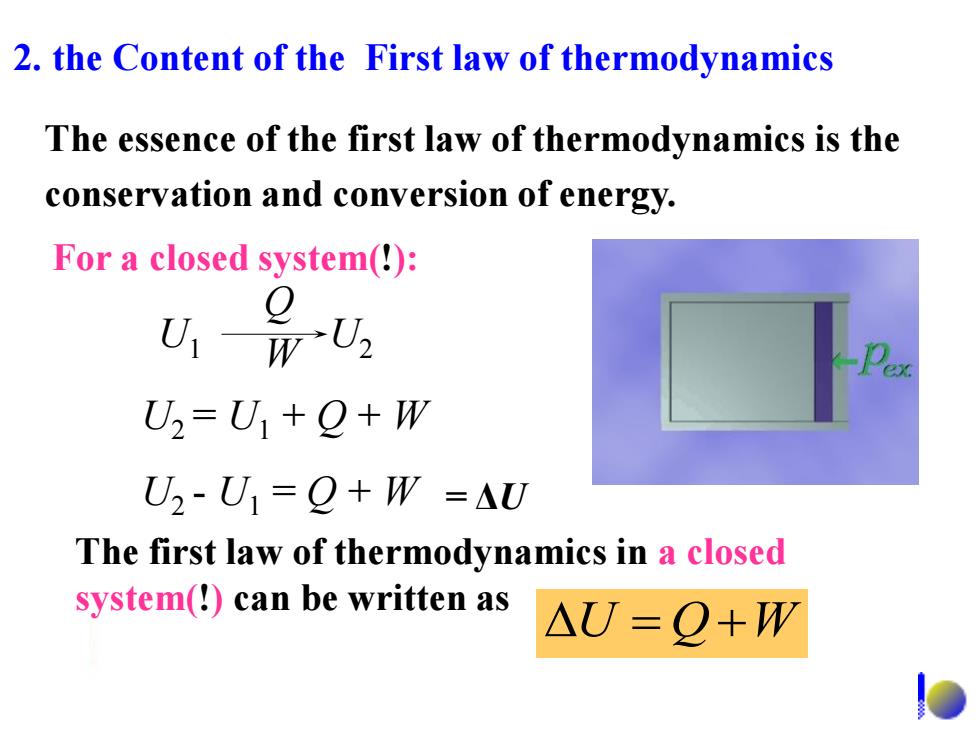
2.the Content of the First law of thermodynamics The essence of the first law of thermodynamics is the conservation and conversion of energy. For a closed system(!): U-w Q U2=U☑1+Q+W U2-U1=Q+W=△U The first law of thermodynamics in a closed system(!)can be written as △U=Q+W
2. the Content of the First law of thermodynamics U QW The first law of thermodynamics in a closed system(!) can be written as The essence of the first law of thermodynamics is the conservation and conversion of energy. U1 U2 Q W U2 = U1 + Q + W U2 - U1 = Q + W = ΔU For a closed system(!):

§2.2 Thermochemistry(热化学) 2.2.1 Heat of a chemical reaction 2.2.2 the Hess's Law 2.2.3 Standard molar enthalpy of formation AHm 2.2.4 Standard molar enthalpy of combustion AH
2.2.1 Heat of a chemical reaction §2.2 Thermochemistry (热化学) 2.2.2 the Hess’s Law 2.2.3 Standard molar enthalpy of formation fHm 2.2.4 Standard molar enthalpy of combustion fHm
2.2.1 Heat of a chemical reaction(化学反应热) △,U=Upodet-Ureactant2+W 热力学第一定律 1.Heat of a reaction at constant volume:O (恒容反应热) Motorzed simer ecical ignting sampe For a closed system of constant volume, Thermomeler nsuated contaner if non-volume work W=0, int Bomb ichamber) Fne ire in with sample Since AV=0,so volume work W=0 Cupgm呢 △UU=O+W The internal energy change of a process is equal to the heat 2,=△U change if it is measured under conditions of constant volume
2.2.1 Heat of a chemical reaction (化学反应热) 1. Heat of a reaction at constant volume: QV For a closed system of constant volume, if non-volume work W = 0, Since V = 0,so volume work W = 0 (恒容反应热) U QW The internal energy change of a process is equal to the heat change if it is measured under conditions of constant volume . Qv = r U r U = Uproduct - Ureactant= Q + W 热力学第一定律