
ammonium salts of oxidizing acids NH4NO2A→N2(g)+2H2O NH4)2Cr,0,4→N2(g+Cr,03S)+4H20 5NH,N0,4N,+2HN0,+9H,0 NH4NO3A→N2O+2H,0 D
ammonium salts of oxidizing acids N H N O N O 2H O 5NH N O 4N 2HNO 9H O (NH ) C r O N (g) C r O (s) 4H O N H N O N (g) 2H O 4 3 2 2 2 3 2 240 C 4 3 4 2 2 7 2 2 3 2 4 2 2 2 催化剂
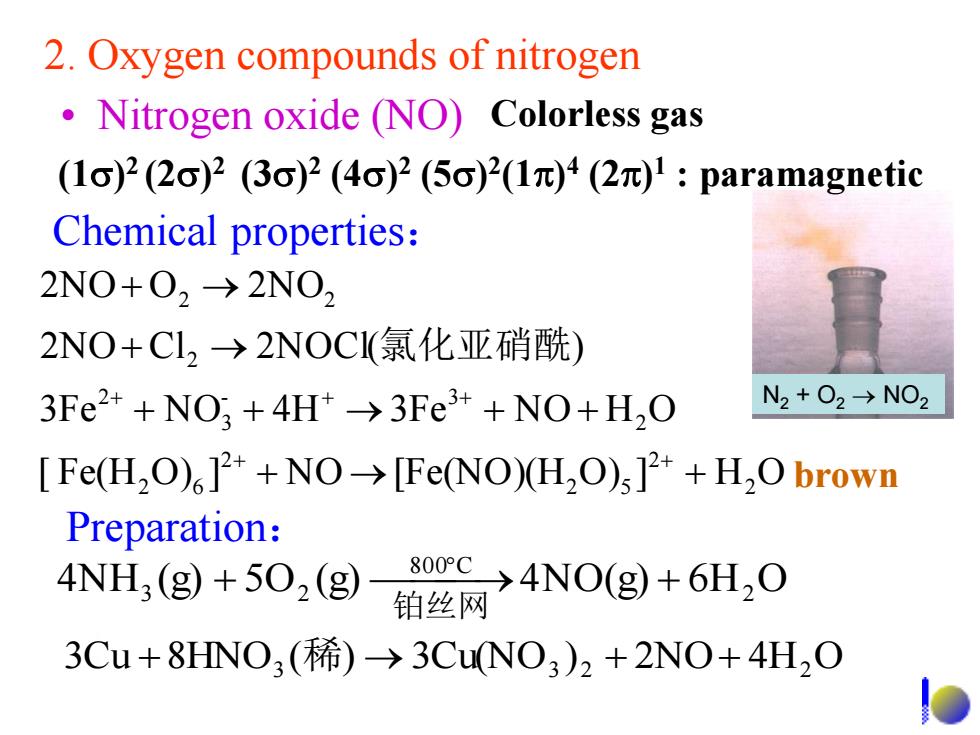
2.Oxygen compounds of nitrogen Nitrogen oxide (NO)Colorless gas (1σ)2(2c)2(3o)2(4o)2(5c)2(1π)4(2)1:paramagnetic Chemical properties: 2NO+O2-→2N02 2NO+Cl,→2NOCl(氯化亚硝酰) 3Fe2++NO;+4H*->3Fe++NO+H,O N2+O2→NO2 Fe(H2O)]2*+NO->[Fe(NO)(H,O)s]2++H2O brown Preparation: 4NH3(g)+502(g) 80oe→4NO(g+6H20 铂丝网 3Cu+8HNO,(稀)→3CuNO3)2+2NO+4H2O
2. Oxygen compounds of nitrogen 3Cu 8HNO3 (稀) 3Cu(NO3 )2 2NO 4H2 O [ Fe(H O ) ] N O [Fe(NO)(H O ) ] H O 3Fe N O 4H 3Fe N O H O 2NO C l 2NOCl( ) 2NO O 2NO 2 2 2 5 2 2 6 2 - 3 3 2 2 2 2 氯化亚硝酰 4NH (g) 5O (g) 4NO(g) 6H2 O 800 C 3 2 铂丝网 • Nitrogen oxide (NO) Preparation: Chemical properties: Colorless gas brown (1) 2 (2) 2 (3) 2 (4) 2 (5) 2 (1) 4 (2) 1 : paramagnetic N2 + O2 NO2

Nitrogen dioxide (NO2) molecular structure: N:2s22p3; sp2 hybridization (a)3个p轨道 Properties: ① toxic,brown gas 2NO,(红棕色)起N,0无色)AH⊙<0 140°C ③ 3NO2+HO-→2HNO3+NO ④ react with bases 2NO,+2NaOH->NaNO3+NaNO,+H2O
• Nitrogen dioxide (NO2 ) N: 2s22p3 ; sp 2 hybridization ① toxic, brown gas ③ 3NO2 H2 O 2HNO3 NO ④ react with bases 2NO2 2NaOH NaNO3 NaNO2 H2 O molecular structure: Properties: ② (无色)Hm 2NO(红棕色) 2 N2O4 <0 冷却 140 C 3 Π3 Π
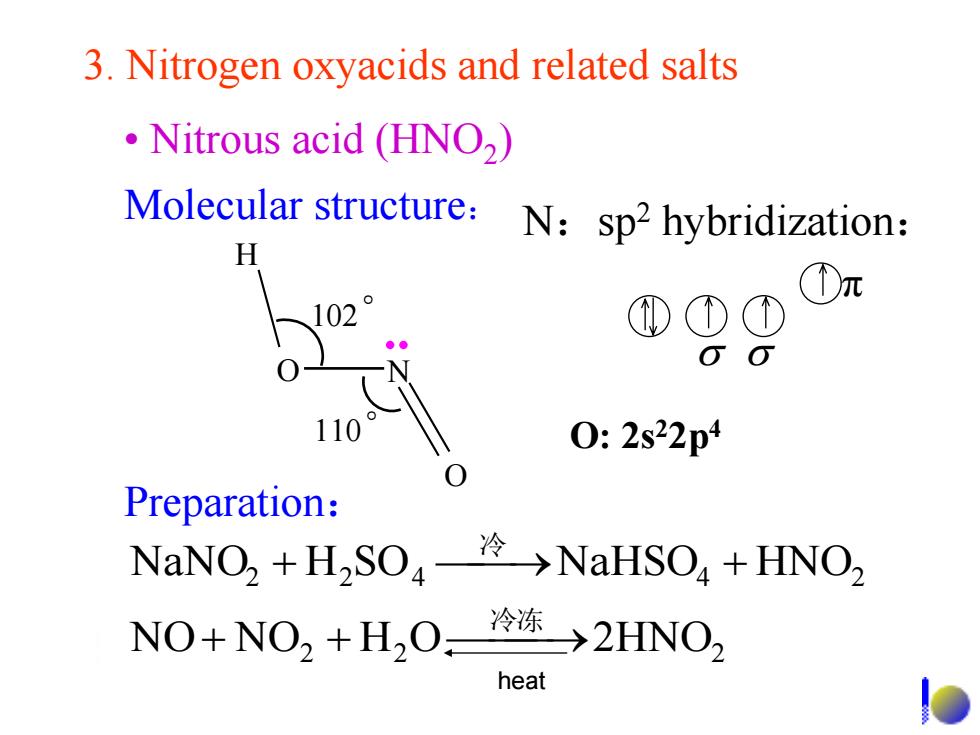
3.Nitrogen oxyacids and related salts Nitrous acid (HNO2) Molecular structure: N:sp2 hybridization: H D元 102 ①①① 110 0:2s22p4 Preparation: NaNO2+H,SO4冷→NaHSO4+HNO2 NO+NO2+H,0冷冻>2HNO, heat
3. Nitrogen oxyacids and related salts N:sp2 hybridization: 2 2 2 2 2 4 4 2 NO NO H O 2HNO NaNO H SO NaHSO HNO 冷冻 冷 π • Nitrous acid (HNO2 ) Preparation: Molecular structure: H O N O 102 。 110 。 heat O: 2s22p4
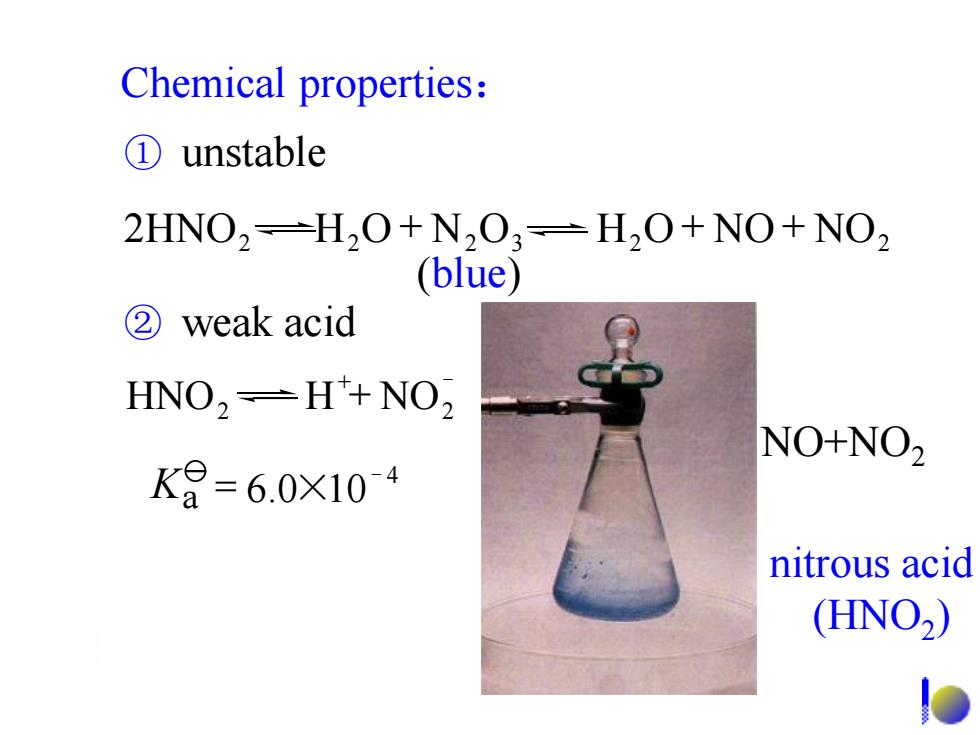
Chemical properties: ①unstable 2HNO2=H20+N203=H20+NO+NO2 (blue) ②weak acid HNO,-H*+NO2 NO+NO2 K8=6.0X104 nitrous acid (HNO2)
nitrous acid (HNO2 ) NO+NO2 Chemical properties: ② weak acid (blue) ① unstable 2 2 _ HNO H NO 4 _ Ka = 6.0×10 2 2 2 3 H2O NO NO2 2HNO H O N O