
·Nitrite(亚硝酸盐) Preparation:treating the acidic oxides with a base NO,+NO+NaOH->2NaNO,+H2O Properties: 1 Most nitrites are colorless and soluble in water (except for AgNO2) ②redox properties (E8(HNO/NO)=1.04V) 2NO +2I+4H 2NO+I,+2H,O 主 NO;+Fe2*+2H*-NO+Fe**+H,O 5NO;+2MnO;+6H*5NO;+2Mn2*+3H,O 3 metals having low activity correspond to nitrites of low thermal stability金属活泼性差,对应亚硝酸盐稳定性差 AgNO2<NaNO2(Mt的极化力)
• Nitrite (亚硝酸盐) Preparation: treating the acidic oxides with a base NO2 NO NaOH 2NaNO2 H2 O Properties: ① Most nitrites are colorless and soluble in water (except for AgNO2 ) ③ metals having low activity correspond to nitrites of low thermal stability 金属活泼性差,对应亚硝酸盐稳定性差 主 AgNO2<NaNO2 (M+的极化力 ) ( (HNO /NO) 1.04V) A 2 E = 2NO 2I 4H 2NO I2 2H2O - - 2 NO Fe 2H NO Fe H2O - 2 3 2 5NO 2MnO 6H 5NO 2Mn 3H2O - 2 3 - 4 - 2 ② redox properties
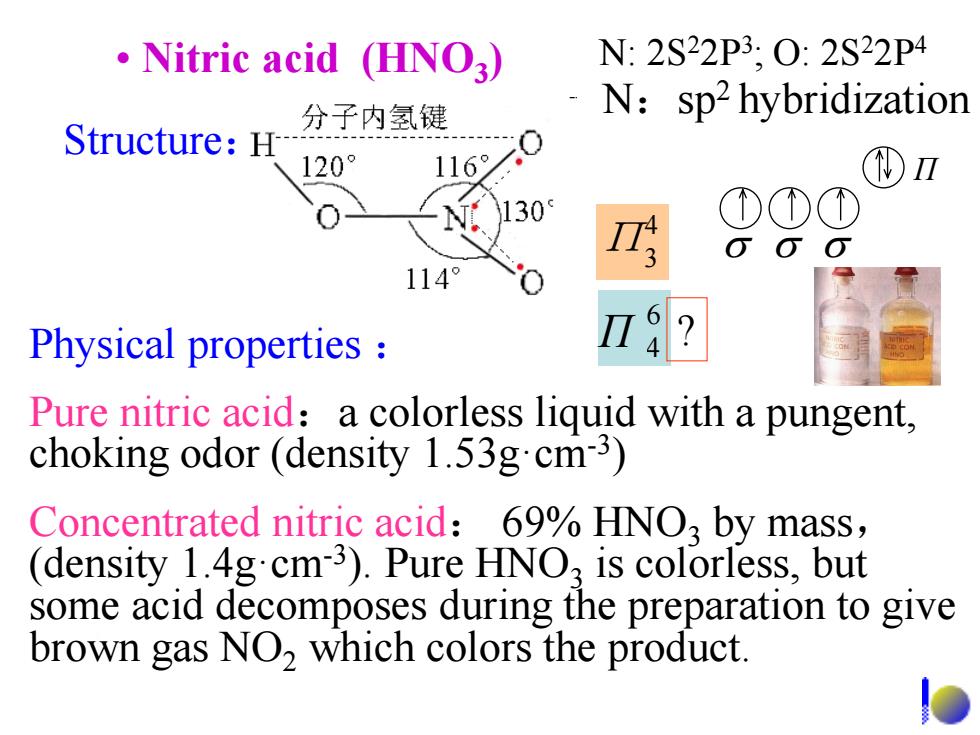
Nitric acid (HNO3) N:2S22P3,O:2S22P4 分子内氢键 N:sp2 hybridization Structure:H 120° 116% 130 114° Physical properties Pure nitric acid:a colorless liquid with a pungent, choking odor (density 1.53g.cm-3) Concentrated nitric acid:69%HNO3 by mass, (density 1.4gcm3).Pure HNO,is colorless,but some acid decomposes during the preparation to give brown gas NO,which colors the product
• Nitric acid (HNO3 ) Physical properties : Pure nitric acid:a colorless liquid with a pungent, choking odor (density 1.53g·cm-3 ) Concentrated nitric acid: 69% HNO3 by mass, (density 1.4g·cm-3 ). Pure HNO3 is colorless, but some acid decomposes during the preparation to give brown gas NO2 which colors the product. 4 Π3 Structure: N:sp2 hybridization Π 6 Π 4 ? N: 2S22P3 ; O: 2S22P4

Chemical properties of HNO3 D Powerful oxidizing agent HNO,+非金属单质→相应高价酸+NO 4HN03+3C→3C02(g)+4NO(g)+2H20 5HNO3+3P+2H,O->3H,PO+5NO(g) 2HNO3+S→H2SO4+2NO 10HN03+3I2→6HO3+10N0+2H2O
Chemical properties of HNO3 ① Powerful oxidizing agent HNO3 非金属单质 相应高价酸 N O 10HNO3 3I2 6HIO3 10NO 2H2 O 2HNO3 S H2 SO4 2NO 5HNO 3P 2H O 3H PO 5NO(g) 3 2 3 4 4HNO3 3C 3CO2 (g) 4NO(g) 2H2 O