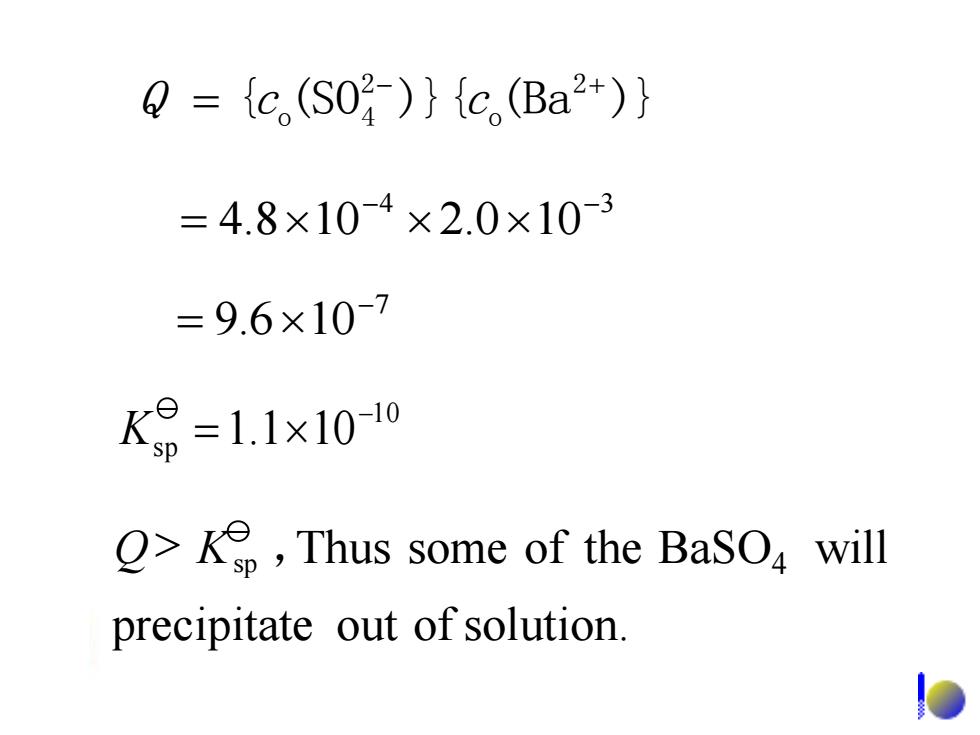
Q={c(S02)}{c.(Ba2+)} =4.8×10-4×2.0×10-3 =9.6×10-7 K9=1.1x10-10 >Ke,Thus some of the BaSO will precipitate out of solution
7 9.6 10- = 4 3 4.8 10 2.0 10 - - = { (SO )}{ (Ba )} 2 o 2 o 4 - + Q = c c 1 0 sp 1.1 10- K = precipitate out of solution. BaSO will 4 Q> Ksp ,Thus some of the
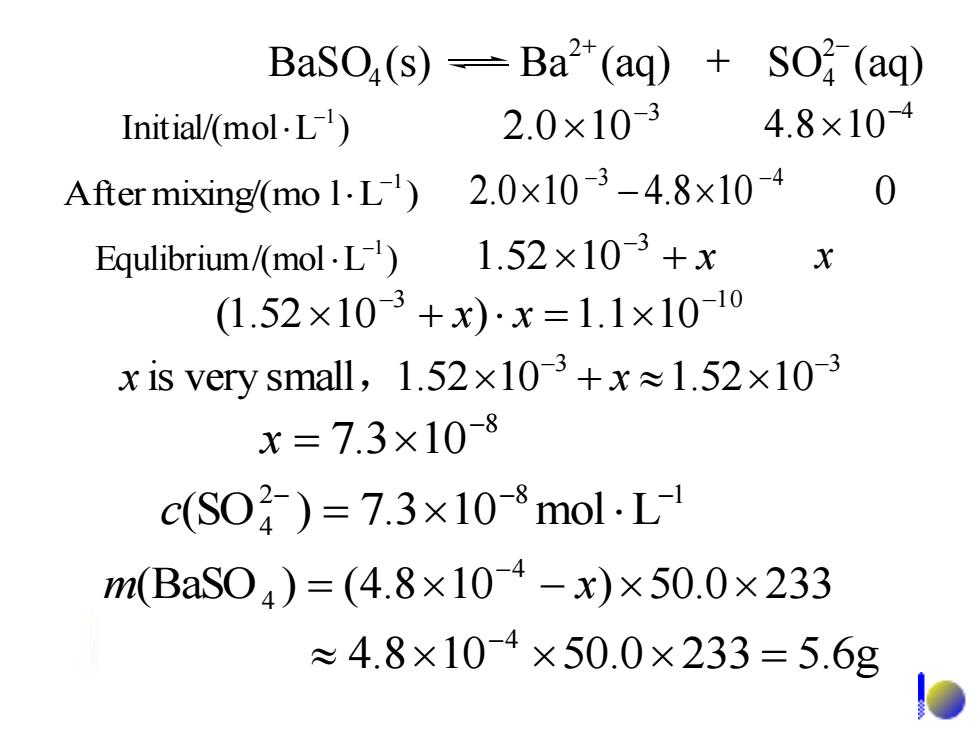
BaSO(s)-Ba2(aq)+SO(aq) Initial/(mol.L 2.0×103 4.8×10-4 After mixing/(mo1.L)2.0x10-3-4.8×l0-4 Equlibrium/(mol.L 1.52x103+x X (1.52×10-3+x)·x=1.1×1010 x is very small,1.52×103+x≈1.52×10-3 x=7.3×108 c(S02)=7.3×10-8molL m(BaS04)=(4.8×104-x)×50.0×233 ≈4.8×10-4×50.0×233=5.6g
3 2.0 10- 4 4.8 10- Initial/(mol L ) -1 3 1 0 (1.52 10 ) 1.1 10 - - + x x = 3 3 is very small 1.52 10 1.52 10 - - x , + x 3 4 2.0 10 4.8 10 - - After mixing/(mo l L ) - 0 -1 + x -3 Equlibrium/(mol L ) 1.52 10 x -1 BaSO (s) Ba (aq) SO (aq) 2 4 2 4 + - + 4.8 10 50.0 233 5.6g 4 = - (BaSO ) (4.8 10 ) 50.0 233 4 4 = - - m x 2 8 1 (SO4 ) 7.3 10 mol L - - - c = 8 7.3 10- x =

9.2.2 The common ion effect and salt effect Common lon Effect 1.The common ion effect The common ion effect is the reduction in the solubility of a sparingly soluble salt by the addition of a soluble salt that has an ion in common with it
9.2.2 The common ion effect and salt effect 1.The common ion effect The common ion effect is the reduction in the solubility of a sparingly soluble salt by the addition of a soluble salt that has an ion in common with it