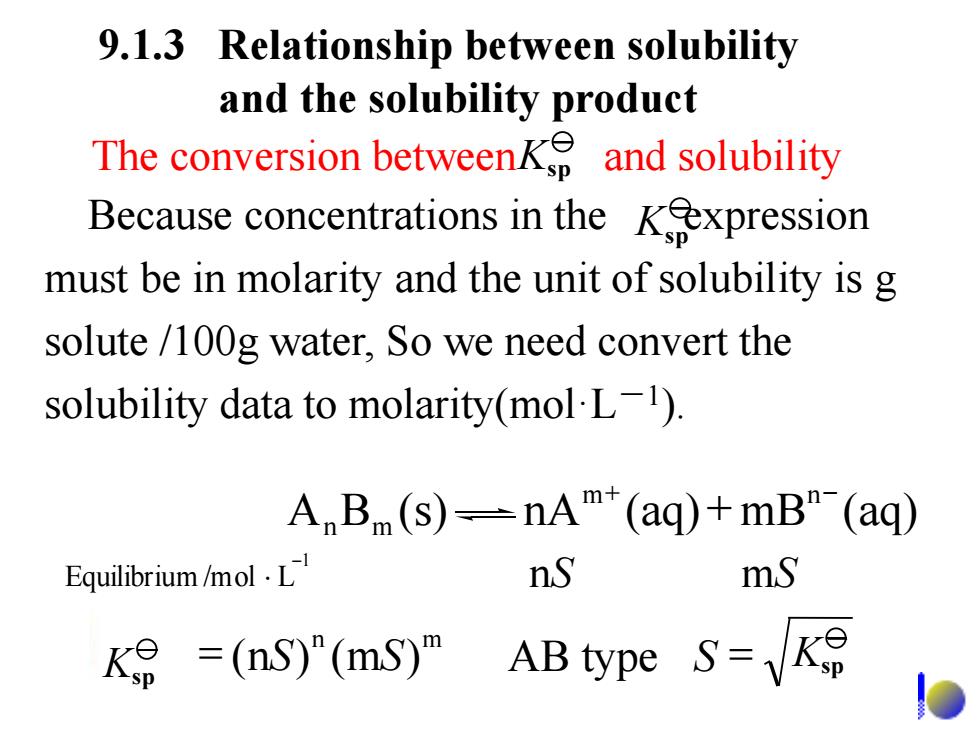
9.1.3 Relationship between solubility and the solubility product The conversion between and solubility Because concentrations in the Kexpression must be in molarity and the unit of solubility is g solute /100g water,So we need convert the solubility data to molarity(mol L). A B(s)--nAT"(aq)+mB"-(aq) Equilibrium /mol·L nS mS =(nS)"(mS)m AB type s=
9.1.3 Relationship between solubility and the solubility product 1 Equilibrium /mol L - nS mS m+ n- A Bm (s) nA (aq)+ mB (aq) n n m K = (nS) (mS) sp AB type S = Ksp The conversion between and solubility Because concentrations in the expression must be in molarity and the unit of solubility is g solute /100g water, So we need convert the solubility data to molarity(mol·L-1 ). Ksp Ksp
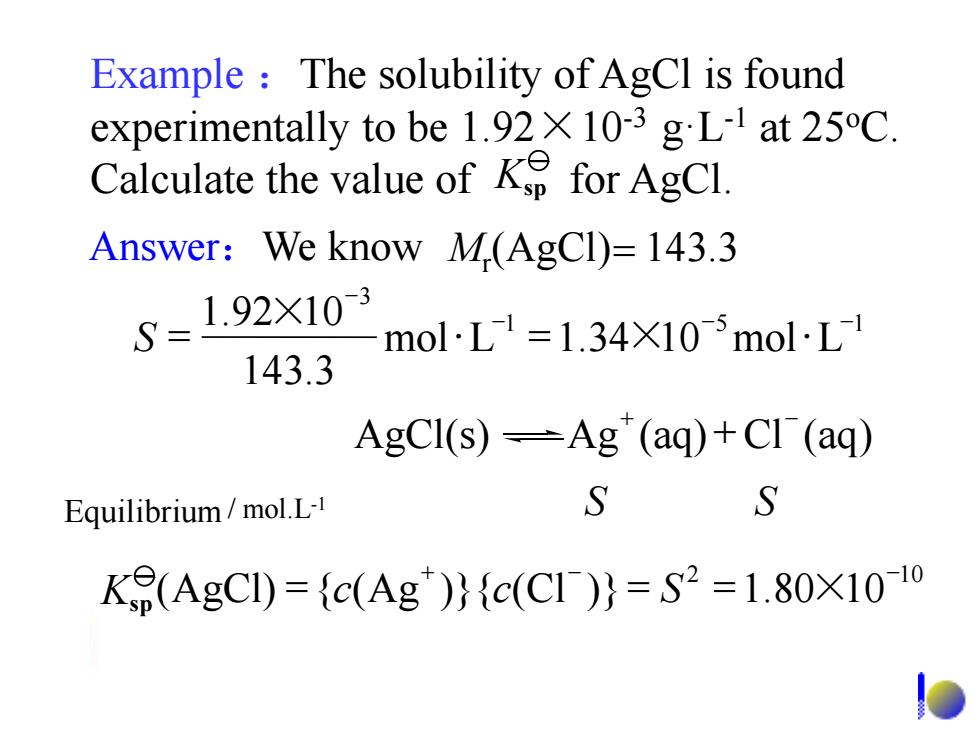
Example The solubility of AgCl is found experimentally to be 1.92X 10-3 gL-1 at 25C. Calculate the value ofK for AgCl. Answer:We know M(AgCl)=143.3 S=1.92X103 molL1=1.34×105molL1 143.3 AgCI(s)=Ag"(aq)+CI (aq) Equilibrium/mol.L- S S K9(AgCI)={c(Ag)}{c(C1)}=S2=1.80X10-1o
Example :The solubility of AgCl is found experimentally to be 1.92×10-3 g·L-1 at 25oC. Calculate the value of for AgCl. Equilibrium / mol.L-1 S S Answer:We know Mr (AgCl)= 143.3 1 5 1 3 mol L 1.34 10 mol L 143.3 1.92 10 - - - - S = × = × AgCl(s) Ag (aq) Cl (aq) + - + 2 10 (AgCl) { (Ag )}{ (Cl )} 1.80 10 + - - Ksp = c c = S = × Ksp

Example:The K for Ag CrO is 1.1X10-12 at 25C.Calculate the solubility of Ag CrO in gL. Answer:Ag2CrOa(s)=2Ag*(aq)+CrO(aq) Equi. /(molL') 2x X Ke(Ag2CrO)=(c(Ag"))2(c(CrO ) 1.1×1012=4x3,x=6.5×105 M(Ag2Cr04)=331.7 S=6.5×105×331.7gL1=2.2×102gL
/(mol L ) 2 1 x x - Equi. Mr (Ag 2CrO4 ) = 331.7 5 1 2 1 6.5 10 331.7 g L 2.2 10 g L - - - - S = × × = × 12 3 5 1.1 10 4 , 6.5 10 - × - × = x x = Ag CrO (s) 2Ag (aq) CrO (aq) 2 4 4 + 2- + + 2- 4 2 2 4 K (Ag CrO ) ={c(Ag )} {c(CrO )} sp Answer: Example:The for Ag2CrO4 is 1.1×10-12 at 25oC. Calculate the solubility of Ag2CrO4 in g·L-1 . Ksp

Question:Determine the relationship between molar solubility (S,unit:mol.L-)and Ke for Ca(PO)2. 5 K S= 108
Question:Determine the relationship between molar solubility (S, unit: mol.L-1 ) and for Ca3 (PO4 )2 . Ksp 5 108 S = Ksp
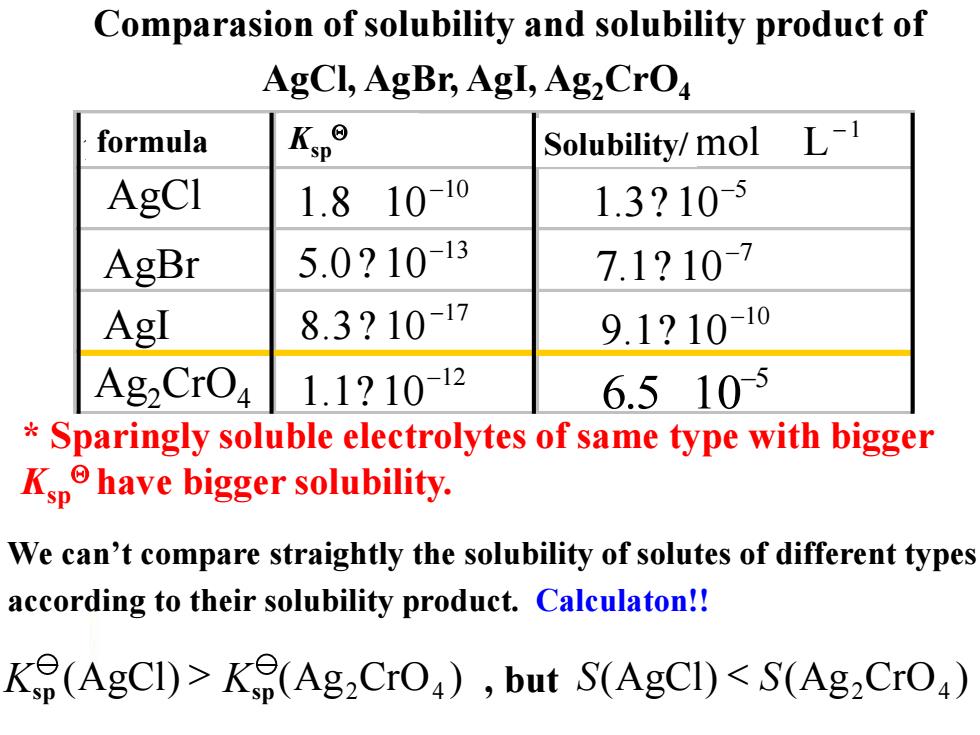
Comparasion of solubility and solubility product of AgCl,AgBr,AgI,Ag2CrO formula Kp° Solubility/mol L-1 AgCI 1.8 10-10 1.3?10-5 AgBr 5.0?10-13 7.1?107 AgI 8.3?10-17 9.1?10-10 AgzCrO 1.1?10-12 6.5105 Sparingly soluble electrolytes of same type with bigger Kphave bigger solubility. We can't compare straightly the solubility of solutes of different types according to their solubility product.Calculaton!! Ke(AgCI)>K(Ag2CrO),but S(AgCI)<S(Ag2CrO)
(AgCl) (Ag CrO ) S S 2 4 < We can’t compare straightly the solubility of solutes of different types according to their solubility product. Calculaton!! * Sparingly soluble electrolytes of same type with bigger Ksp have bigger solubility. (AgCl) (Ag CrO ) 2 4 K > sp Ksp Comparasion of solubility and solubility product of AgCl, AgBr, AgI, Ag2CrO4 , but 分子式 溶度积 溶解度/ AgBr AgI AgCl 5 6.5 10- 1 mol L - 10 1.8 10- 13 5.0 10- ? 17 8.3 10 - ? 12 1.1 10- ? 10 9.1 10- ? 7 7.1 10- ? 5 1.3 10- ? Ag2CrO4 formula Ksp Solubility/