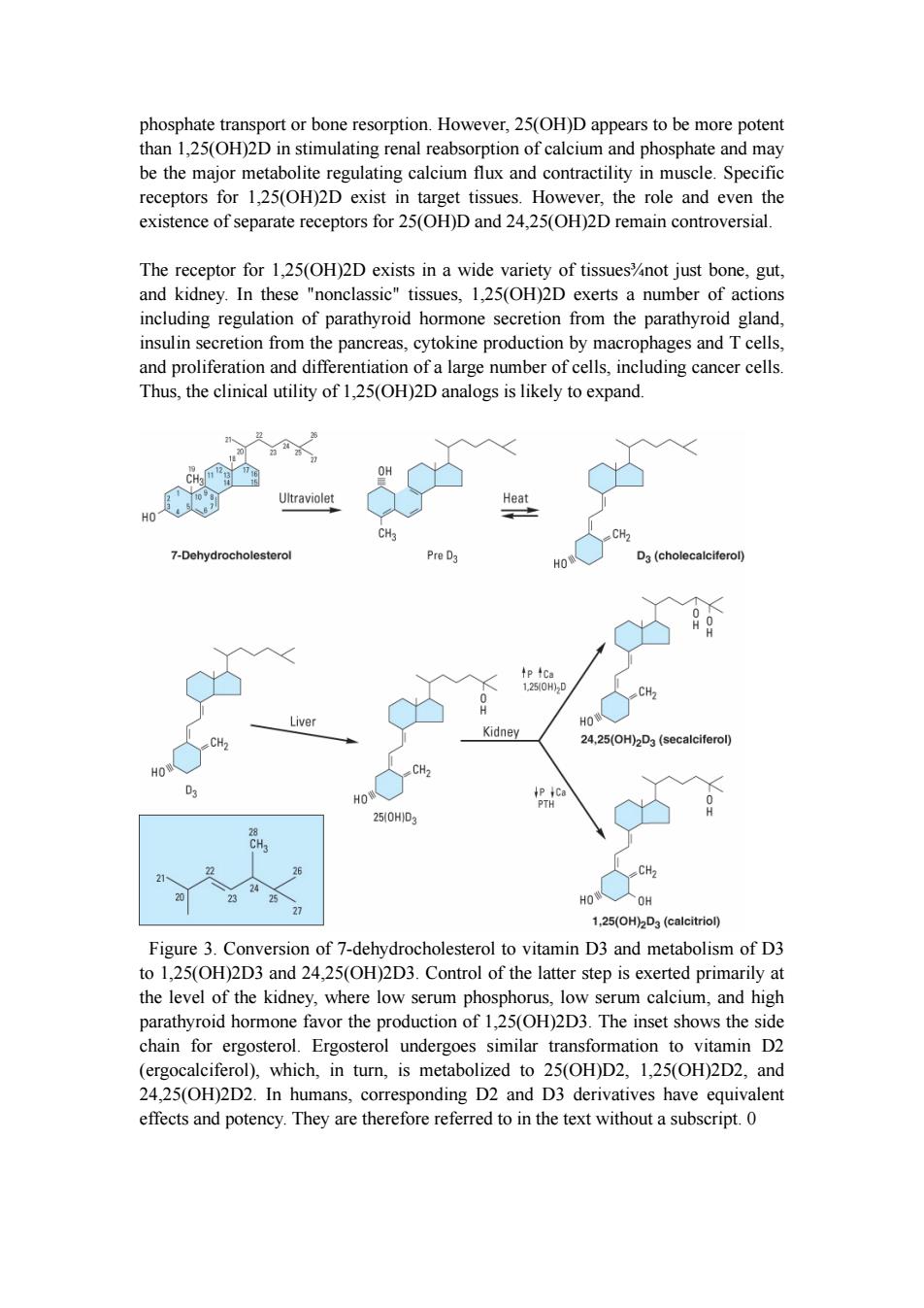
phosphate transport or bone resorption.However,25(OH)D appears to be more potent than 1,25(OH)2D in stimulating renal reabsorption of calcium and phosphate and may be the major metabolite regulating calcium flux and contractility in muscle.Specific receptors for 1,25(OH)2D exist in target tissues.However,the role and even the existence of separate receptors for 25(OH)D and 24,25(OH)2D remain controversial. The receptor for 1,25(OH)2D exists in a wide variety of tissues%not just bone,gut, and kidney.In these "nonclassic"tissues,1,25(OH)2D exerts a number of actions including regulation of parathyroid hormone secretion from the parathyroid gland, insulin secretion from the pancreas,cytokine production by macrophages and T cells, and proliferation and differentiation of a large number of cells,including cancer cells. Thus,the clinical utility of 1,25(OH)2D analogs is likely to expand 10 Ultraviolet Heat CH3 CH2 7-Dehydrocholesterol Pre D3 HO D3(cholecalciferol) tp4Ca 1250H)2D CH2 Liver HO Kidney CH. 24,25(OH)2D3(secalciferol) HO HO 250HD3 CH2 HO人OH 27 1,25(OH)2D3 (calcitriol) Figure 3.Conversion of 7-dehydrocholesterol to vitamin D3 and metabolism of D3 to 1,25(OH)2D3 and 24,25(OH)2D3.Control of the latter step is exerted primarily at the level of the kidney,where low serum phosphorus,low serum calcium,and high parathyroid hormone favor the production of 1,25(OH)2D3.The inset shows the side chain for ergosterol.Ergosterol undergoes similar transformation to vitamin D2 (ergocalciferol),which,in turn,is metabolized to 25(OH)D2,1,25(OH)2D2,and 24,25(OH)2D2.In humans,corresponding D2 and D3 derivatives have equivalent effects and potency.They are therefore referred to in the text without a subscript.0
phosphate transport or bone resorption. However, 25(OH)D appears to be more potent than 1,25(OH)2D in stimulating renal reabsorption of calcium and phosphate and may be the major metabolite regulating calcium flux and contractility in muscle. Specific receptors for 1,25(OH)2D exist in target tissues. However, the role and even the existence of separate receptors for 25(OH)D and 24,25(OH)2D remain controversial. The receptor for 1,25(OH)2D exists in a wide variety of tissues¾not just bone, gut, and kidney. In these "nonclassic" tissues, 1,25(OH)2D exerts a number of actions including regulation of parathyroid hormone secretion from the parathyroid gland, insulin secretion from the pancreas, cytokine production by macrophages and T cells, and proliferation and differentiation of a large number of cells, including cancer cells. Thus, the clinical utility of 1,25(OH)2D analogs is likely to expand. Figure 3. Conversion of 7-dehydrocholesterol to vitamin D3 and metabolism of D3 to 1,25(OH)2D3 and 24,25(OH)2D3. Control of the latter step is exerted primarily at the level of the kidney, where low serum phosphorus, low serum calcium, and high parathyroid hormone favor the production of 1,25(OH)2D3. The inset shows the side chain for ergosterol. Ergosterol undergoes similar transformation to vitamin D2 (ergocalciferol), which, in turn, is metabolized to 25(OH)D2, 1,25(OH)2D2, and 24,25(OH)2D2. In humans, corresponding D2 and D3 derivatives have equivalent effects and potency. They are therefore referred to in the text without a subscript. 0

INTERACTION OF PTH VITAMIN D A summary of the principal actions of PTH and vitamin D on the three main target tissues%intestine,kidney,and bone%is presented in Table 42-2.The net effect of PTH is to raise serum calcium and reduce serum phosphate;the net effect of vitamin D is to raise both.Regulation of calcium and phosphate homeostasis is achieved through a variety of feedback loops.Calcium is the principal regulator of PTH secretion.It binds to a novel ion recognition site that is part of a Gq protein-coupled receptor called the calcium sensing receptor (CaR)and links changes in intracellular free calcium concentration to changes in extracellular calcium.As serum calcium levels rise and bind to this receptor,intracellular calcium levels increase and inhibit PTH secretion.Phosphate regulates PTH secretion directly and indirectly by forming complexes with calcium in the serum.Because it is the ionized free concentration of calcium that is detected by the parathyroid gland,increases in serum phosphate levels reduce the ionized calcium and lead to enhanced PTH secretion.Such feedback regulation is appropriate to the net effect of PTH to raise serum calcium and reduce serum phosphate levels.Likewise,both calcium and phosphate at high levels reduce the amount of 1,25(OH)2D produced by the kidney and increase the amount of 24,25(OH)2D produced.The high calcium works directly and indirectly by reducing PTH secretion.The high phosphate works directly and indirectly by increasing FGF23 levels.Since 1,25(OH)2D raises serum calcium and phosphate,whereas 24,25(OH)2D has less effect,such feedback regulation is again appropriate. 1,25(OH)2D itself directly inhibits PTH secretion (independently of its effect on serum calcium)by a direct action on PTH gene transcription.This provides yet another negative feedback loop.The ability of 1,25(OH)2D to inhibit PTH secretion directly is being exploited using calcitriol analogs that have less effect on serum calcium because of their lesser effect on intestinal calcium absorption.Such drugs are proving useful in the management of secondary hyperparathyroidism accompanying chronic kidney disease and may be useful in selected cases of primary hyperparathyroidism. SECONDARY HORMONAL REGULATORS OF BONE MINERAL HOMEOSTASIS INTRODUCTION A number of hormones modulate the actions of PTH and vitamin D in regulating bone mineral homeostasis.Compared with that of PTH and vitamin D,the physiologic impact of such secondary regulation on bone mineral homeostasis is minor.However, in pharmacologic amounts,a number of these hormones have actions on the bone mineral homeostatic mechanisms that can be exploited therapeutically. CALCITONIN
INTERACTION OF PTH & VITAMIN D A summary of the principal actions of PTH and vitamin D on the three main target tissues¾intestine, kidney, and bone¾is presented in Table 42-2. The net effect of PTH is to raise serum calcium and reduce serum phosphate; the net effect of vitamin D is to raise both. Regulation of calcium and phosphate homeostasis is achieved through a variety of feedback loops. Calcium is the principal regulator of PTH secretion. It binds to a novel ion recognition site that is part of a Gq protein-coupled receptor called the calcium sensing receptor (CaR) and links changes in intracellular free calcium concentration to changes in extracellular calcium. As serum calcium levels rise and bind to this receptor, intracellular calcium levels increase and inhibit PTH secretion. Phosphate regulates PTH secretion directly and indirectly by forming complexes with calcium in the serum. Because it is the ionized free concentration of calcium that is detected by the parathyroid gland, increases in serum phosphate levels reduce the ionized calcium and lead to enhanced PTH secretion. Such feedback regulation is appropriate to the net effect of PTH to raise serum calcium and reduce serum phosphate levels. Likewise, both calcium and phosphate at high levels reduce the amount of 1,25(OH)2D produced by the kidney and increase the amount of 24,25(OH)2D produced. The high calcium works directly and indirectly by reducing PTH secretion. The high phosphate works directly and indirectly by increasing FGF23 levels. Since 1,25(OH)2D raises serum calcium and phosphate, whereas 24,25(OH)2D has less effect, such feedback regulation is again appropriate. 1,25(OH)2D itself directly inhibits PTH secretion (independently of its effect on serum calcium) by a direct action on PTH gene transcription. This provides yet another negative feedback loop. The ability of 1,25(OH)2D to inhibit PTH secretion directly is being exploited using calcitriol analogs that have less effect on serum calcium because of their lesser effect on intestinal calcium absorption. Such drugs are proving useful in the management of secondary hyperparathyroidism accompanying chronic kidney disease and may be useful in selected cases of primary hyperparathyroidism. SECONDARY HORMONAL REGULATORS OF BONE MINERAL HOMEOSTASIS INTRODUCTION A number of hormones modulate the actions of PTH and vitamin D in regulating bone mineral homeostasis. Compared with that of PTH and vitamin D, the physiologic impact of such secondary regulation on bone mineral homeostasis is minor. However, in pharmacologic amounts, a number of these hormones have actions on the bone mineral homeostatic mechanisms that can be exploited therapeutically. CALCITONIN

The calcitonin secreted by the parafollicular cells of the mammalian thyroid is a single-chain peptide hormone with 32 amino acids and a molecular weight of 3600.A disulfide bond between positions 1 and 7 is essential for biologic activity.Calcitonin is produced from a precursor with MW 15,000.The circulating forms of calcitonin are multiple,ranging in size from the monomer (MW 3600)to forms with an apparent molecular weight of 60,000.Whether such heterogeneity includes precursor forms or covalently linked oligomers is not known.Because of its heterogeneity,calcitonin is standardized by bioassay in rats.Activity is compared to a standard maintained by the British Medical Research Council(MRC)and expressed as MRC units. Human calcitonin monomer has a half-life of about 10 minutes with a metabolic clearance of 8-9 mL/kg/min.Salmon calcitonin has a longer half-life and a reduced metabolic clearance(3 mL/kg/min),making it more attractive as a therapeutic agent Much of the clearance occurs in the kidney,although little intact calcitonin appears in the urine The principal effects of calcitonin are to lower serum calcium and phosphate by actions on bone and kidney.Calcitonin inhibits osteoclastic bone resorption.Although bone formation is not impaired at first after calcitonin administration,with time both formation and resorption of bone are reduced.In the kidney,calcitonin reduces both calcium and phosphate reabsorption as well as reabsorption of other ions,including sodium,potassium,and magnesium.Tissues other than bone and kidney are also affected by calcitonin.Calcitonin in pharmacologic amounts decreases gastrin secretion and reduces gastric acid output while increasing secretion of sodium, potassium,chloride,and water in the gut.Pentagastrin is a potent stimulator of calcitonin secretion (as is hypercalcemia),suggesting a possible physiologic relationship between gastrin and calcitonin.In the adult human,no readily demonstrable problem develops in cases of calcitonin deficiency (thyroidectomy)or excess (medullary carcinoma of the thyroid).However,the ability of calcitonin to block bone resorption and lower serum calcium makes it a useful drug for the treatment of Paget's disease,hypercalcemia,and osteoporosis. GLUCOCORTICOIDS Glucocorticoid hormones alter bone mineral homeostasis by antagonizing vitamin D-stimulated intestinal calcium transport,by stimulating renal calcium excretion,and by blocking bone formation.Although these observations underscore the negative impact of glucocorticoids on bone mineral homeostasis,these hormones have proved useful in reversing the hypercalcemia associated with lymphomas and granulomatous diseases such as sarcoidosis (in which production of 1,25[OH]2D is increased)or in cases of vitamin D intoxication.Prolonged administration of glucocorticoids is a common cause of osteoporosis in adults and stunted skeletal development in children. ESTROGENS
The calcitonin secreted by the parafollicular cells of the mammalian thyroid is a single-chain peptide hormone with 32 amino acids and a molecular weight of 3600. A disulfide bond between positions 1 and 7 is essential for biologic activity. Calcitonin is produced from a precursor with MW 15,000. The circulating forms of calcitonin are multiple, ranging in size from the monomer (MW 3600) to forms with an apparent molecular weight of 60,000. Whether such heterogeneity includes precursor forms or covalently linked oligomers is not known. Because of its heterogeneity, calcitonin is standardized by bioassay in rats. Activity is compared to a standard maintained by the British Medical Research Council (MRC) and expressed as MRC units. Human calcitonin monomer has a half-life of about 10 minutes with a metabolic clearance of 8-9 mL/kg/min. Salmon calcitonin has a longer half-life and a reduced metabolic clearance (3 mL/kg/min), making it more attractive as a therapeutic agent. Much of the clearance occurs in the kidney, although little intact calcitonin appears in the urine. The principal effects of calcitonin are to lower serum calcium and phosphate by actions on bone and kidney. Calcitonin inhibits osteoclastic bone resorption. Although bone formation is not impaired at first after calcitonin administration, with time both formation and resorption of bone are reduced. In the kidney, calcitonin reduces both calcium and phosphate reabsorption as well as reabsorption of other ions, including sodium, potassium, and magnesium. Tissues other than bone and kidney are also affected by calcitonin. Calcitonin in pharmacologic amounts decreases gastrin secretion and reduces gastric acid output while increasing secretion of sodium, potassium, chloride, and water in the gut. Pentagastrin is a potent stimulator of calcitonin secretion (as is hypercalcemia), suggesting a possible physiologic relationship between gastrin and calcitonin. In the adult human, no readily demonstrable problem develops in cases of calcitonin deficiency (thyroidectomy) or excess (medullary carcinoma of the thyroid). However, the ability of calcitonin to block bone resorption and lower serum calcium makes it a useful drug for the treatment of Paget's disease, hypercalcemia, and osteoporosis. GLUCOCORTICOIDS Glucocorticoid hormones alter bone mineral homeostasis by antagonizing vitamin D-stimulated intestinal calcium transport, by stimulating renal calcium excretion, and by blocking bone formation. Although these observations underscore the negative impact of glucocorticoids on bone mineral homeostasis, these hormones have proved useful in reversing the hypercalcemia associated with lymphomas and granulomatous diseases such as sarcoidosis (in which production of 1,25[OH]2D is increased) or in cases of vitamin D intoxication. Prolonged administration of glucocorticoids is a common cause of osteoporosis in adults and stunted skeletal development in children. ESTROGENS

Estrogens can prevent accelerated bone loss during the immediate postmenopausal period and at least transiently increase bone in the postmenopausal woman.The prevailing hypothesis advanced to explain these observations is that estrogens reduce the bone-resorbing action of PTH.Estrogen administration leads to an increased 1,25(OH)2D level in blood,but estrogens have no direct effect on 1,25(OH)2D production in vitro.The increased 1,25(OH)2D levels in vivo following estrogen treatment may result from decreased serum calcium and phosphate and increased PTH. Estrogen receptors have been found in bone,and estrogen has direct effects on bone remodeling.Recent case reports of men who lack the estrogen receptor or who are unable to produce estrogen because of aromatase deficiency noted marked osteopenia and failure to close epiphyses.This further substantiates the role of estrogen in bone development,even in men.The principal therapeutic application for estrogen administration in disorders of bone mineral homeostasis is the treatment or prevention of postmenopausal osteoporosis.However,long-term use of estrogen is being discouraged because of its deleterious side effects.Rather,selective estrogen receptor modulators(SERMs)have been developed to retain the beneficial effects on bone while minimizing these deleterious side effects on breast,uterus,and the cardiovascular system (see Box:Newer Therapies for Osteoporosis). NEWER THERAPIES FOR OSTEOPOROSIS Bone undergoes a continuous remodeling process involving bone resorption and formation.Any process that disrupts this balance by increasing resorption relative to formation results in osteoporosis.Inadequate sex hormone production is a major cause of osteoporosis in men and women.Estrogen replacement therapy at menopause is a well-established means of preventing osteoporosis in the female,but many women fear its adverse effects,particularly the increased risk of breast cancer from continued estrogen use (the well-demonstrated increased risk of endometrial cancer is prevented by cycling with a progestin)and do not like the persistence of menstrual bleeding that often accompanies this form of therapy.Medical enthusiasm for this treatment has waned with the demonstration that it does not protect against heart disease. Raloxifene is the first of the selective estrogen receptor modulators (SERMs;see Chapter 40)to be approved for the prevention of osteoporosis.Raloxifene shares some of the beneficial effects of estrogen on bone without increasing the risk of breast or endometrial cancer (it may actually reduce the risk of breast cancer).Although not as effective as estrogen in increasing bone density,raloxifene has been shown to reduce vertebral fractures. Nonhormonal forms of therapy for osteoporosis with proven efficacy in reducing fracture risk have also been developed.Bisphosphonates such as alendronate, risedronate,and ibandronate have been conclusively shown to increase bone density and reduce fractures over at least 5 years when used continuously at a dosage of 10
Estrogens can prevent accelerated bone loss during the immediate postmenopausal period and at least transiently increase bone in the postmenopausal woman. The prevailing hypothesis advanced to explain these observations is that estrogens reduce the bone-resorbing action of PTH. Estrogen administration leads to an increased 1,25(OH)2D level in blood, but estrogens have no direct effect on 1,25(OH)2D production in vitro. The increased 1,25(OH)2D levels in vivo following estrogen treatment may result from decreased serum calcium and phosphate and increased PTH. Estrogen receptors have been found in bone, and estrogen has direct effects on bone remodeling. Recent case reports of men who lack the estrogen receptor or who are unable to produce estrogen because of aromatase deficiency noted marked osteopenia and failure to close epiphyses. This further substantiates the role of estrogen in bone development, even in men. The principal therapeutic application for estrogen administration in disorders of bone mineral homeostasis is the treatment or prevention of postmenopausal osteoporosis. However, long-term use of estrogen is being discouraged because of its deleterious side effects. Rather, selective estrogen receptor modulators (SERMs) have been developed to retain the beneficial effects on bone while minimizing these deleterious side effects on breast, uterus, and the cardiovascular system (see Box: Newer Therapies for Osteoporosis). NEWER THERAPIES FOR OSTEOPOROSIS Bone undergoes a continuous remodeling process involving bone resorption and formation. Any process that disrupts this balance by increasing resorption relative to formation results in osteoporosis. Inadequate sex hormone production is a major cause of osteoporosis in men and women. Estrogen replacement therapy at menopause is a well-established means of preventing osteoporosis in the female, but many women fear its adverse effects, particularly the increased risk of breast cancer from continued estrogen use (the well-demonstrated increased risk of endometrial cancer is prevented by cycling with a progestin) and do not like the persistence of menstrual bleeding that often accompanies this form of therapy. Medical enthusiasm for this treatment has waned with the demonstration that it does not protect against heart disease. Raloxifene is the first of the selective estrogen receptor modulators (SERMs; see Chapter 40) to be approved for the prevention of osteoporosis. Raloxifene shares some of the beneficial effects of estrogen on bone without increasing the risk of breast or endometrial cancer (it may actually reduce the risk of breast cancer). Although not as effective as estrogen in increasing bone density, raloxifene has been shown to reduce vertebral fractures. Nonhormonal forms of therapy for osteoporosis with proven efficacy in reducing fracture risk have also been developed. Bisphosphonates such as alendronate, risedronate, and ibandronate have been conclusively shown to increase bone density and reduce fractures over at least 5 years when used continuously at a dosage of 10

mg/d or 70 mg/wk for alendronate and 5 mg/d or 35 mg/wk for risedronate,2.5 mg/d or 150 mg/mo for ibandronate.Side-by-side trials between alendronate and calcitonin (another approved nonestrogen drug for osteoporosis)indicated a greater efficacy of alendronate.Bisphosphonates are poorly absorbed and must be given on an empty stomach or infused intravenously.At the higher oral doses used in the treatment of Paget's disease,alendronate causes gastric irritation,but this is not a significant problem at the doses recommended for osteoporosis when patients are instructed to take the drug with a glass of water and remain upright.The most recently approved drug for osteoporosis is teriparatide,the recombinant form of PTH1-34.Unlike other approved drugs for osteoporosis,teriparatide stimulates bone formation rather than inhibiting bone resorption.However,teriparatide must be given daily by subcutaneous injection.Its efficacy in preventing fractures appears to be at least as great as that of the bisphosphonates.In all cases,adequate intake of calcium and vitamin D needs to be maintained. Thus,we now have several well-validated,efficacious forms of treatment for this common debilitating disease. NONHORMONALAGENTS AFFECTING BONE MINERAL HOMEOSTASIS BISPHOSPHONATES The bisphosphonates are analogs of pyrophosphate in which the P-O-P bond has been replaced with a nonhydrolyzable P-C-P bond(Figure 4).Etidronate,pamidronate,and alendronate have now been joined by risedronate,tiludronate,ibandronate,and zoledronate for clinical use.The bisphosphonates owe at least part of their clinical usefulness and toxicity to their ability to retard formation and dissolution of hydroxyapatite crystals within and outside the skeletal system.They localize to regions of bone resorption and so exert their greatest effects on osteoclasts.However, the exact mechanism by which they selectively inhibit bone resorption is not clear. The results from animal and clinical studies indicate that less than 10%of an oral dose of these drugs is absorbed.Food reduces absorption even further,necessitating their administration on an empty stomach.Because it causes gastric irritation, pamidronate is not available as an oral preparation.However,with the possible exception of etidronate,all currently available bisphosphonates have this complication. Nearly half of the absorbed drug accumulates in bone;the remainder is excreted unchanged in the urine.Decreased renal function,esophageal motility disorders,and peptic ulcer disease are the main contraindications to the use of these drugs.The portion bound to bone is retained for months,depending on the turnover of bone itself. Etidronate and the other bisphosphonates exert a variety of effects on bone mineral homeostasis.In particular,bisphosphonates are useful for the treatment of
mg/d or 70 mg/wk for alendronate and 5 mg/d or 35 mg/wk for risedronate, 2.5 mg/d or 150 mg/mo for ibandronate. Side-by-side trials between alendronate and calcitonin (another approved nonestrogen drug for osteoporosis) indicated a greater efficacy of alendronate. Bisphosphonates are poorly absorbed and must be given on an empty stomach or infused intravenously. At the higher oral doses used in the treatment of Paget's disease, alendronate causes gastric irritation, but this is not a significant problem at the doses recommended for osteoporosis when patients are instructed to take the drug with a glass of water and remain upright. The most recently approved drug for osteoporosis is teriparatide, the recombinant form of PTH1-34. Unlike other approved drugs for osteoporosis, teriparatide stimulates bone formation rather than inhibiting bone resorption. However, teriparatide must be given daily by subcutaneous injection. Its efficacy in preventing fractures appears to be at least as great as that of the bisphosphonates. In all cases, adequate intake of calcium and vitamin D needs to be maintained. Thus, we now have several well-validated, efficacious forms of treatment for this common debilitating disease. NONHORMONAL AGENTS AFFECTING BONE MINERAL HOMEOSTASIS BISPHOSPHONATES The bisphosphonates are analogs of pyrophosphate in which the P-O-P bond has been replaced with a nonhydrolyzable P-C-P bond (Figure 4). Etidronate, pamidronate, and alendronate have now been joined by risedronate, tiludronate, ibandronate, and zoledronate for clinical use. The bisphosphonates owe at least part of their clinical usefulness and toxicity to their ability to retard formation and dissolution of hydroxyapatite crystals within and outside the skeletal system. They localize to regions of bone resorption and so exert their greatest effects on osteoclasts. However, the exact mechanism by which they selectively inhibit bone resorption is not clear. The results from animal and clinical studies indicate that less than 10% of an oral dose of these drugs is absorbed. Food reduces absorption even further, necessitating their administration on an empty stomach. Because it causes gastric irritation, pamidronate is not available as an oral preparation. However, with the possible exception of etidronate, all currently available bisphosphonates have this complication. Nearly half of the absorbed drug accumulates in bone; the remainder is excreted unchanged in the urine. Decreased renal function, esophageal motility disorders, and peptic ulcer disease are the main contraindications to the use of these drugs. The portion bound to bone is retained for months, depending on the turnover of bone itself. Etidronate and the other bisphosphonates exert a variety of effects on bone mineral homeostasis. In particular, bisphosphonates are useful for the treatment of