
4.Drug Biotransformation Maria Almira Correia,PhD INTRODUCTION Humans are exposed daily to a wide variety of foreign compounds called xenobiotics%4substances absorbed across the lungs or skin or,more commonly, ingested either unintentionally as compounds present in food and drink or deliberately as drugs for therapeutic or "recreational"purposes.Exposure to environmental xenobiotics may be inadvertent and accidental or%when they are present as components of air,water,and foodinescapable.Some xenobiotics are innocuous, but many can provoke biologic responses.Such biologic responses often depend on conversion of the absorbed substance into an active metabolite.The discussion that follows is applicable to xenobiotics in general(including drugs)and to some extent to endogenous compounds. WHY IS DRUG BIOTRANSFORMATION NECESSARY? Renal excretion plays a pivotal role in terminating the biologic activity of some drugs, particularly those that have small molecular volumes or possess polar characteristics such as functional groups that are fully ionized at physiologic pH.However,many drugs do not possess such physicochemical properties.Pharmacologically active organic molecules tend to be lipophilic and remain unionized or only partially ionized at physiologic pH;these are readily reabsorbed from the glomerular filtrate in the nephron.Certain lipophilic compounds are often strongly bound to plasma proteins and may not be readily filtered at the glomerulus.Consequently,most drugs would have a prolonged duration of action if termination of their action depended solely on renal excretion. An alternative process that can lead to the termination or alteration of biologic activity is metabolism.In general,lipophilic xenobiotics are transformed to more polar and hence more readily excreted products.The role metabolism plays in the inactivation of lipid-soluble drugs can be quite dramatic.For example,lipophilic barbiturates such as thiopental and pentobarbital would have extremely long half-lives if it were not for their metabolic conversion to more water-soluble compounds. Metabolic products are often less pharmacodynamically active than the parent drug and may even be inactive.However,some biotransformation products have enhanced activity or toxic properties.It is noteworthy that the synthesis of endogenous substrates such as steroid hormones,cholesterol,active vitamin D congeners,and bile acids involves many pathways catalyzed by enzymes associated with the metabolism of xenobiotics.Finally,drug-metabolizing enzymes have been exploited in the design of pharmacologically inactive prodrugs that are converted to active molecules in the body
4. Drug Biotransformation ¾ Maria Almira Correia, PhD INTRODUCTION Humans are exposed daily to a wide variety of foreign compounds called xenobiotics¾substances absorbed across the lungs or skin or, more commonly, ingested either unintentionally as compounds present in food and drink or deliberately as drugs for therapeutic or "recreational" purposes. Exposure to environmental xenobiotics may be inadvertent and accidental or¾when they are present as components of air, water, and food¾inescapable. Some xenobiotics are innocuous, but many can provoke biologic responses. Such biologic responses often depend on conversion of the absorbed substance into an active metabolite. The discussion that follows is applicable to xenobiotics in general (including drugs) and to some extent to endogenous compounds. WHY IS DRUG BIOTRANSFORMATION NECESSARY? Renal excretion plays a pivotal role in terminating the biologic activity of some drugs, particularly those that have small molecular volumes or possess polar characteristics such as functional groups that are fully ionized at physiologic pH. However, many drugs do not possess such physicochemical properties. Pharmacologically active organic molecules tend to be lipophilic and remain unionized or only partially ionized at physiologic pH; these are readily reabsorbed from the glomerular filtrate in the nephron. Certain lipophilic compounds are often strongly bound to plasma proteins and may not be readily filtered at the glomerulus. Consequently, most drugs would have a prolonged duration of action if termination of their action depended solely on renal excretion. An alternative process that can lead to the termination or alteration of biologic activity is metabolism. In general, lipophilic xenobiotics are transformed to more polar and hence more readily excreted products. The role metabolism plays in the inactivation of lipid-soluble drugs can be quite dramatic. For example, lipophilic barbiturates such as thiopental and pentobarbital would have extremely long half-lives if it were not for their metabolic conversion to more water-soluble compounds. Metabolic products are often less pharmacodynamically active than the parent drug and may even be inactive. However, some biotransformation products have enhanced activity or toxic properties. It is noteworthy that the synthesis of endogenous substrates such as steroid hormones, cholesterol, active vitamin D congeners, and bile acids involves many pathways catalyzed by enzymes associated with the metabolism of xenobiotics. Finally, drug-metabolizing enzymes have been exploited in the design of pharmacologically inactive prodrugs that are converted to active molecules in the body

THE ROLE OF BIOTRANSFORMATION IN DRUG DISPOSITION Most metabolic biotransformations occur at some point between absorption of the drug into the general circulation and its renal elimination.A few transformations occur in the intestinal lumen or intestinal wall.In general,all of these reactions can be assigned to one of two major categories called phase I and phase II reactions Figure 4-1). Phase I reactions usually convert the parent drug to a more polar metabolite by introducing or unmasking a functional group(-OH,-NH2,-SH).Often these metabolites are inactive,although in some instances activity is only modified or even enhanced. If phase I metabolites are sufficiently polar,they may be readily excreted.However, many phase I products are not eliminated rapidly and undergo a subsequent reaction in which an endogenous substrate such as glucuronic acid,sulfuric acid,acetic acid, or an amino acid combines with the newly incorporated functional group to form a highly polar conjugate.Such conjugation or synthetic reactions are the hallmarks of phase II metabolism.A great variety of drugs undergo these sequential biotransformation reactions,although in some instances the parent drug may already possess a functional group that may form a conjugate directly.For example,the hydrazide moiety of isoniazid is known to form an N-acetyl conjugate in a phase II reaction.This conjugate is then a substrate for a phase I type reaction,namely, hydrolysis to isonicotinic acid(Figure 4-2).Thus,phase II reactions may actually precede phase I reactions. ABSORPTION METABOLISM ELIMINATION Phase l Phase ll Drug Conjugate Drug metabolite with modified activity Conjugate Drug Inactive drug metabolite Conjugate Drug Lipophilic Hydrophilic
THE ROLE OF BIOTRANSFORMATION IN DRUG DISPOSITION Most metabolic biotransformations occur at some point between absorption of the drug into the general circulation and its renal elimination. A few transformations occur in the intestinal lumen or intestinal wall. In general, all of these reactions can be assigned to one of two major categories called phase I and phase II reactions ( Figure 4-1). Phase I reactions usually convert the parent drug to a more polar metabolite by introducing or unmasking a functional group (-OH, -NH2, -SH). Often these metabolites are inactive, although in some instances activity is only modified or even enhanced. If phase I metabolites are sufficiently polar, they may be readily excreted. However, many phase I products are not eliminated rapidly and undergo a subsequent reaction in which an endogenous substrate such as glucuronic acid, sulfuric acid, acetic acid, or an amino acid combines with the newly incorporated functional group to form a highly polar conjugate. Such conjugation or synthetic reactions are the hallmarks of phase II metabolism. A great variety of drugs undergo these sequential biotransformation reactions, although in some instances the parent drug may already possess a functional group that may form a conjugate directly. For example, the hydrazide moiety of isoniazid is known to form an N-acetyl conjugate in a phase II reaction. This conjugate is then a substrate for a phase I type reaction, namely, hydrolysis to isonicotinic acid (Figure 4-2). Thus, phase II reactions may actually precede phase I reactions

0 H C-N一NH2 (INH) Phase ll(acetylation) 0 H H 0 C-N一N-C- CH3 (N-acetyl INH) Phase I(hydrolysis) 0 0H C- OH +CH3-C-N-NH2(acetylhydrazine) Acetylation of Isonicotinic acid macromolecules (proteins) Hepatotoxicity Figure 4-1.Phase I and phase II reactions,and direct elimination,in drug biodisposition.Phase II reactions may also precede phase I reactions. Figure 4-2.Phase II activation of isoniazid(INH)to a hepatotoxic metabolite. WHERE DO DRUG BIOTRANSFORMATIONS OCCUR? Although every tissue has some ability to metabolize drugs,the liver is the principal organ of drug metabolism.Other tissues that display considerable activity include the gastrointestinal tract,the lungs,the skin,and the kidneys.Following oral administration,many drugs(eg,isoproterenol,meperidine,pentazocine,morphine) are absorbed intact from the small intestine and transported first via the portal system to the liver,where they undergo extensive metabolism.This process is called the first-pass effect(see Chapter 3).Some orally administered drugs (eg,clonazepam, chlorpromazine,cyclosporine)are more extensively metabolized in the intestine than in the liver,whereas others (eg,midazolam)undergo significant(50%)intestinal metabolism.Thus,intestinal metabolism can contribute to the overall first-pass effect, and individuals with compromised liver function may increasingly rely on such intestinal metabolism for drug elimination.First-pass effects may so greatly limit the bioavailability of orally administered drugs (eg,lidocaine)that alternative routes of administration must be used to achieve therapeutically effective blood levels. Furthermore,the lower gut harbors intestinal microorganisms that are capable of
Figure 4-1. Phase I and phase II reactions, and direct elimination, in drug biodisposition. Phase II reactions may also precede phase I reactions. Figure 4-2. Phase II activation of isoniazid (INH) to a hepatotoxic metabolite. WHERE DO DRUG BIOTRANSFORMATIONS OCCUR? Although every tissue has some ability to metabolize drugs, the liver is the principal organ of drug metabolism. Other tissues that display considerable activity include the gastrointestinal tract, the lungs, the skin, and the kidneys. Following oral administration, many drugs (eg, isoproterenol, meperidine, pentazocine, morphine) are absorbed intact from the small intestine and transported first via the portal system to the liver, where they undergo extensive metabolism. This process is called the first-pass effect (see Chapter 3). Some orally administered drugs (eg, clonazepam, chlorpromazine, cyclosporine) are more extensively metabolized in the intestine than in the liver, whereas others (eg, midazolam) undergo significant (50%) intestinal metabolism. Thus, intestinal metabolism can contribute to the overall first-pass effect, and individuals with compromised liver function may increasingly rely on such intestinal metabolism for drug elimination. First-pass effects may so greatly limit the bioavailability of orally administered drugs (eg, lidocaine) that alternative routes of administration must be used to achieve therapeutically effective blood levels. Furthermore, the lower gut harbors intestinal microorganisms that are capable of

many biotransformation reactions.In addition,drugs may be metabolized by gastric acid(eg,penicillin),by digestive enzymes(eg,polypeptides such as insulin),or by enzymes in the wall of the intestine (eg,sympathomimetic catecholamines) Although drug biotransformation in vivo can occur by spontaneous,noncatalyzed chemical reactions,the vast majority of transformations are catalyzed by specific cellular enzymes.At the subcellular level,these enzymes may be located in the endoplasmic reticulum (ER).mitochondria.cytosol,lysosomes.or even the nuclear envelope or plasma membrane. MICROSOMAL MIXED FUNCTION OXIDASE SYSTEM PHASE I REACTIONS Introduction Many drug-metabolizing enzymes are located in the lipophilic ER membranes of the liver and other tissues.When these lamellar membranes are isolated by homogenization and fractionation of the cell,they re-form into vesicles called microsomes.Microsomes retain most of the morphologic and functional characteristics of the intact membranes,including the rough and smooth surface features of the rough(ribosome-studded)and smooth(no ribosomes)ER.Whereas the rough microsomes tend to be dedicated to protein synthesis,the smooth microsomes are relatively rich in enzymes responsible for oxidative drug metabolism.In particular, they contain the important class of enzymes known as the mixed function oxidases (MFOs),or monooxygenases.The activity of these enzymes requires both a reducing agent (nicotinamide adenine dinucleotide phosphate [NADPH])and molecular oxygen;in a typical reaction,one molecule of oxygen is consumed(reduced)per substrate molecule,with one oxygen atom appearing in the product and the other in the form of water. In this oxidation-reduction process,two microsomal enzymes play a key role.The first of these is a flavoprotein,NADPH-cytochrome P450 reductase.One mole of this enzyme contains 1 mol each of flavin mononucleotide (FMN)and flavin adenine dinucleotide(FAD).The second microsomal enzyme is a hemoprotein called cytochrome P450,which serves as the terminal oxidase.In fact,the microsomal membrane harbors multiple forms of this hemoprotein,and this multiplicity is increased by repeated administration of exogenous chemicals(see below).The name cytochrome P450(abbreviated as CYP or P450)is derived from the spectral properties of this hemoprotein.In its reduced (ferrous)form,it binds carbon monoxide to give a complex that absorbs light maximally at 450 nm.The relative abundance of P450s,compared with that of the reductase in the liver,contributes to making P450 heme reduction a rate-limiting step in hepatic drug oxidations. Microsomal drug oxidations require P450,P450 reductase,NADPH,and molecular
many biotransformation reactions. In addition, drugs may be metabolized by gastric acid (eg, penicillin), by digestive enzymes (eg, polypeptides such as insulin), or by enzymes in the wall of the intestine (eg, sympathomimetic catecholamines). Although drug biotransformation in vivo can occur by spontaneous, noncatalyzed chemical reactions, the vast majority of transformations are catalyzed by specific cellular enzymes. At the subcellular level, these enzymes may be located in the endoplasmic reticulum (ER), mitochondria, cytosol, lysosomes, or even the nuclear envelope or plasma membrane. MICROSOMAL MIXED FUNCTION OXIDASE SYSTEM & PHASE I REACTIONS Introduction Many drug-metabolizing enzymes are located in the lipophilic ER membranes of the liver and other tissues. When these lamellar membranes are isolated by homogenization and fractionation of the cell, they re-form into vesicles called microsomes. Microsomes retain most of the morphologic and functional characteristics of the intact membranes, including the rough and smooth surface features of the rough (ribosome-studded) and smooth (no ribosomes) ER. Whereas the rough microsomes tend to be dedicated to protein synthesis, the smooth microsomes are relatively rich in enzymes responsible for oxidative drug metabolism. In particular, they contain the important class of enzymes known as the mixed function oxidases (MFOs), or monooxygenases. The activity of these enzymes requires both a reducing agent (nicotinamide adenine dinucleotide phosphate [NADPH]) and molecular oxygen; in a typical reaction, one molecule of oxygen is consumed (reduced) per substrate molecule, with one oxygen atom appearing in the product and the other in the form of water. In this oxidation-reduction process, two microsomal enzymes play a key role. The first of these is a flavoprotein, NADPH-cytochrome P450 reductase. One mole of this enzyme contains 1 mol each of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). The second microsomal enzyme is a hemoprotein called cytochrome P450, which serves as the terminal oxidase. In fact, the microsomal membrane harbors multiple forms of this hemoprotein, and this multiplicity is increased by repeated administration of exogenous chemicals (see below). The name cytochrome P450 (abbreviated as CYP or P450) is derived from the spectral properties of this hemoprotein. In its reduced (ferrous) form, it binds carbon monoxide to give a complex that absorbs light maximally at 450 nm. The relative abundance of P450s, compared with that of the reductase in the liver, contributes to making P450 heme reduction a rate-limiting step in hepatic drug oxidations. Microsomal drug oxidations require P450, P450 reductase, NADPH, and molecular
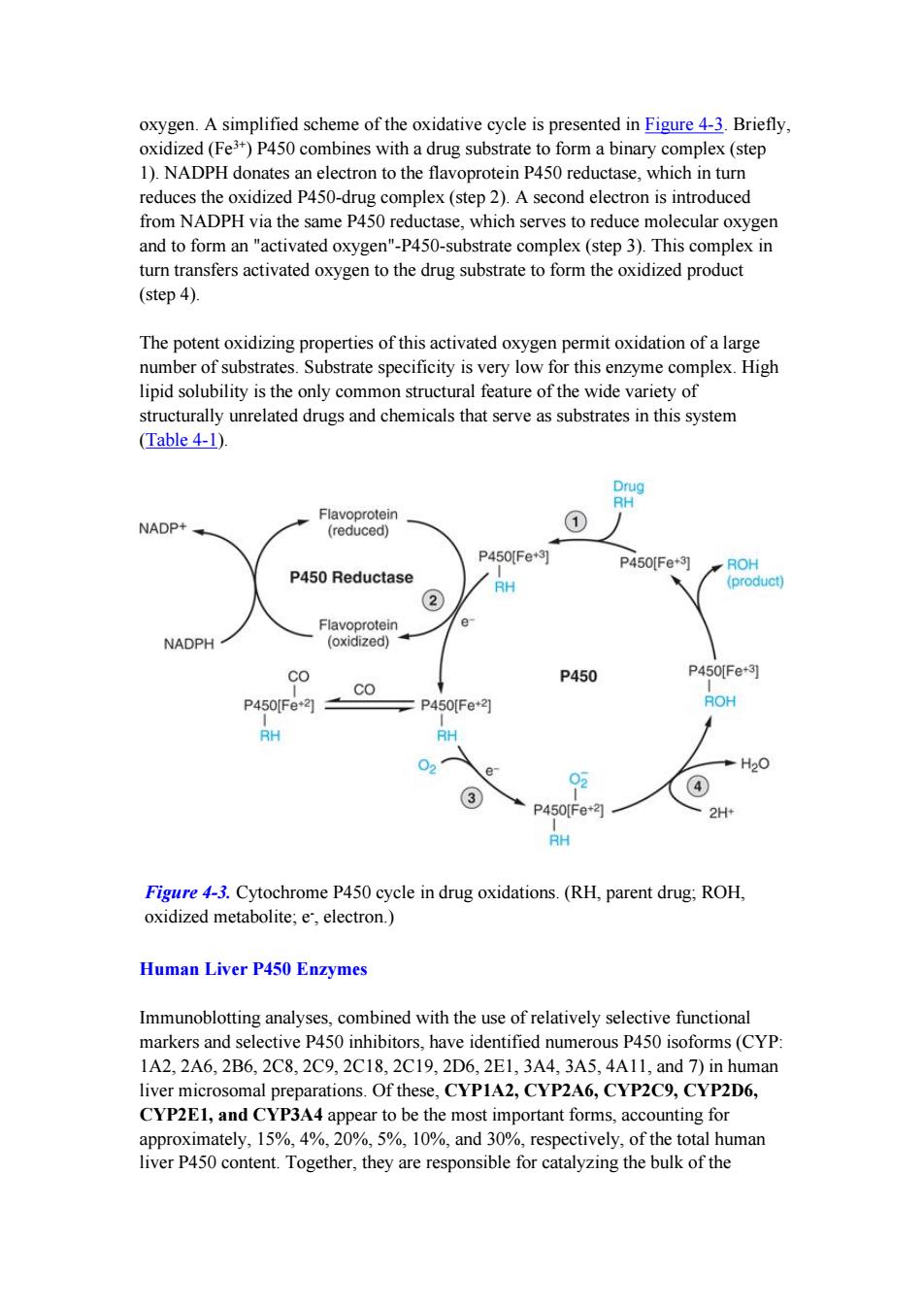
oxygen.A simplified scheme of the oxidative cycle is presented in Figure 4-3.Briefly, oxidized(Fe3+)P450 combines with a drug substrate to form a binary complex(step 1).NADPH donates an electron to the flavoprotein P450 reductase,which in turn reduces the oxidized P450-drug complex(step 2).A second electron is introduced from NADPH via the same P450 reductase,which serves to reduce molecular oxygen and to form an "activated oxygen"-P450-substrate complex (step 3).This complex in turn transfers activated oxygen to the drug substrate to form the oxidized product (step 4). The potent oxidizing properties of this activated oxygen permit oxidation of a large number of substrates.Substrate specificity is very low for this enzyme complex.High lipid solubility is the only common structural feature of the wide variety of structurally unrelated drugs and chemicals that serve as substrates in this system (Table 4-1). Drug RH Flavoprotein NADP+ (reduced) P450Fe+3] P450[Fe+3] ROH P450 Reductase RH (product) ② Flavoprotein e NADPH (oxidized) co P450 P450Fe+3 CO P450[Fe21 P450Fe+2 ROH RH RH H20 02 4 P450[Fe*3 2H+ RH Figure 4-3.Cytochrome P450 cycle in drug oxidations.(RH,parent drug;ROH, oxidized metabolite;e,electron.) Human Liver P450 Enzymes Immunoblotting analyses,combined with the use of relatively selective functional markers and selective P450 inhibitors,have identified numerous P450 isoforms(CYP: 1A2,2A6,2B6,2C8,2C9,2C18,2C19,2D6,2E1,3A4,3A5,4A11,and7)in human liver microsomal preparations.Of these,CYP1A2,CYP2A6,CYP2C9,CYP2D6, CYP2E1,and CYP3A4 appear to be the most important forms,accounting for approximately,15%,4%,20%,5%,10%,and 30%,respectively,of the total human liver P450 content.Together,they are responsible for catalyzing the bulk of the
oxygen. A simplified scheme of the oxidative cycle is presented in Figure 4-3. Briefly, oxidized (Fe 3+ ) P450 combines with a drug substrate to form a binary complex (step 1). NADPH donates an electron to the flavoprotein P450 reductase, which in turn reduces the oxidized P450-drug complex (step 2). A second electron is introduced from NADPH via the same P450 reductase, which serves to reduce molecular oxygen and to form an "activated oxygen"-P450-substrate complex (step 3). This complex in turn transfers activated oxygen to the drug substrate to form the oxidized product (step 4). The potent oxidizing properties of this activated oxygen permit oxidation of a large number of substrates. Substrate specificity is very low for this enzyme complex. High lipid solubility is the only common structural feature of the wide variety of structurally unrelated drugs and chemicals that serve as substrates in this system (Table 4-1). Figure 4-3. Cytochrome P450 cycle in drug oxidations. (RH, parent drug; ROH, oxidized metabolite; e -, electron.) Human Liver P450 Enzymes Immunoblotting analyses, combined with the use of relatively selective functional markers and selective P450 inhibitors, have identified numerous P450 isoforms (CYP: 1A2, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, 4A11, and 7) in human liver microsomal preparations. Of these, CYP1A2, CYP2A6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 appear to be the most important forms, accounting for approximately, 15%, 4%, 20%, 5%, 10%, and 30%, respectively, of the total human liver P450 content. Together, they are responsible for catalyzing the bulk of the