
Antiseizure Drugs Roger J.Porter,MD,Brian S.Meldrum,MB,PhD Introduction Approximately 1%of the world's population has epilepsy,the second most common neurologic disorder after stroke.Although standard therapy permits control of seizures in 80%of these patients,millions (500,000 people in the USA alone)have uncontrolled epilepsy.Epilepsy is a heterogeneous symptom complex%a chronic disorder characterized by recurrent seizures.Seizures are finite episodes of brain dysfunction resulting from abnormal discharge of cerebral neurons.The causes of seizures are many and include the full range of neurologic diseases%from infection to neoplasm and head injury.In some subgroups,heredity has proved to be a predominant factor. The antiseizure drugs described in this chapter are also used in patients with febrile seizures or with seizures occurring as part of an acute illness such as meningitis.The term "epilepsy"is not usually applied to such patients unless chronic seizures develop later.Seizures are occasionally caused by an acute underlying toxic or metabolic disorder,in which case appropriate therapy should be directed toward the specific abnormality,eg,hypocalcemia.In most cases of epilepsy,however,the choice of medication depends on the empiric seizure classification. Drug Development for Epilepsy For a long time it was assumed that a single drug could be developed for the treatment of all forms of epilepsy,but the causes of epilepsy are extremely diverse, encompassing genetic and developmental defects and infective,traumatic,neoplastic, and degenerative disease processes.Drug therapy to date shows little evidence of etiologic specificity.There is,however,some specificity according to seizure type. This is most clearly seen with generalized seizures of the absence type.These are typically seen with 2-3 Hz spike-and-wave discharges on the electroencephalogram, which respond to ethosuximide and valproate but can be exacerbated by phenytoin and carbamazepine.Drugs acting selectively on absence seizures can be identified by animal screens,using either threshold pentylenetetrazol clonic seizures in mice or rats or mutant mice showing absence-like episodes (so-called lethargic,stargazer,or tottering mutants).In contrast,the maximal electroshock(MES)test,with suppression of the tonic extensor phase,identifies drugs such as phenytoin,carbamazepine,and lamotrigine that are active against generalized tonic-clonic seizures and complex partial seizures.Use of the maximal electroshock test as the major initial screen for new drugs has probably led to the identification of drugs with a common mechanism of action involving prolonged inactivation of the voltage-sensitive sodium channel. Limbic seizures induced in rats by the process of electrical kindling (involving
Antiseizure Drugs Roger J. Porter, MD, & Brian S. Meldrum, MB, PhD Introduction Approximately 1% of the world's population has epilepsy, the second most common neurologic disorder after stroke. Although standard therapy permits control of seizures in 80% of these patients, millions (500,000 people in the USA alone) have uncontrolled epilepsy. Epilepsy is a heterogeneous symptom complex¾a chronic disorder characterized by recurrent seizures. Seizures are finite episodes of brain dysfunction resulting from abnormal discharge of cerebral neurons. The causes of seizures are many and include the full range of neurologic diseases¾from infection to neoplasm and head injury. In some subgroups, heredity has proved to be a predominant factor. The antiseizure drugs described in this chapter are also used in patients with febrile seizures or with seizures occurring as part of an acute illness such as meningitis. The term "epilepsy" is not usually applied to such patients unless chronic seizures develop later. Seizures are occasionally caused by an acute underlying toxic or metabolic disorder, in which case appropriate therapy should be directed toward the specific abnormality, eg, hypocalcemia. In most cases of epilepsy, however, the choice of medication depends on the empiric seizure classification. Drug Development for Epilepsy For a long time it was assumed that a single drug could be developed for the treatment of all forms of epilepsy, but the causes of epilepsy are extremely diverse, encompassing genetic and developmental defects and infective, traumatic, neoplastic, and degenerative disease processes. Drug therapy to date shows little evidence of etiologic specificity. There is, however, some specificity according to seizure type. This is most clearly seen with generalized seizures of the absence type. These are typically seen with 2-3 Hz spike-and-wave discharges on the electroencephalogram, which respond to ethosuximide and valproate but can be exacerbated by phenytoin and carbamazepine. Drugs acting selectively on absence seizures can be identified by animal screens, using either threshold pentylenetetrazol clonic seizures in mice or rats or mutant mice showing absence-like episodes (so-called lethargic, stargazer, or tottering mutants). In contrast, the maximal electroshock (MES) test, with suppression of the tonic extensor phase, identifies drugs such as phenytoin, carbamazepine, and lamotrigine that are active against generalized tonic-clonic seizures and complex partial seizures. Use of the maximal electroshock test as the major initial screen for new drugs has probably led to the identification of drugs with a common mechanism of action involving prolonged inactivation of the voltage-sensitive sodium channel. Limbic seizures induced in rats by the process of electrical kindling (involving

repeated episodes of focal electrical stimulation)probably provide a better screen for predicting efficacy in complex partial seizures. Existing antiseizure drugs provide adequate seizure control in about two thirds of patients.A fraction of the epileptic population is resistant to all available drugs,and this may be due to increased expression of the multidrug transporter P-glycoprotein 170,a product of the ABCBI gene.In children,some severe seizure syndromes associated with progressive brain damage are very difficult to treat.In adults,some focal seizures are refractory to medications.Some,particularly in the temporal lobe, are amenable to surgical resection.Some of the drug-resistant population may respond to vagus-nerve stimulation(VNS),a nonpharmacologic treatment for epilepsy now widely approved for treatment of patients with partial seizures.VNS is indicated for refractory cases or for patients in whom antiseizure drugs are poorly tolerated. Stimulating electrodes are implanted in the left vagus nerve,and the pacemaker is implanted in the chest wall or axilla.Use of this device may permit seizure control with lower doses of drugs. New antiseizure drugs are being sought not only by the screening tests noted above but also by more rational approaches.Compounds are sought that act by one of three mechanisms:(1)enhancement of GABAergic (inhibitory)transmission,(2) diminution of excitatory (usually glutamatergic)transmission,or (3)modification of ionic conductances. I.BASIC PHARMACOLOGY OF ANTISEIZURE DRUGS Chemistry Until 1990,approximately 16 antiseizure drugs were available,and 13 of them can be classified into five very similar chemical groups:barbiturates,hydantoins, oxazolidinediones,succinimides,and acetylureas.These groups have in common a similar heterocyclic ring structure with a variety of substituents(Figure 24-1).For drugs with this basic structure,the substituents on the heterocyclic ring determine the pharmacologic class,either anti-MES or antipentylenetetrazol.Very small changes in structure can dramatically alter the mechanism of action and clinical properties of the compound.The remaining drugs%4carbamazepine,valproic acid,and the benzodiazepines%are structurally dissimilar,as are the newer compounds marketed since 1990,ie,felbamate,gabapentin,lamotrigine,levetiracetam,oxcarbazepine, pregabalin,tiagabine,topiramate,vigabatrin,and zonisamide
repeated episodes of focal electrical stimulation) probably provide a better screen for predicting efficacy in complex partial seizures. Existing antiseizure drugs provide adequate seizure control in about two thirds of patients. A fraction of the epileptic population is resistant to all available drugs, and this may be due to increased expression of the multidrug transporter P-glycoprotein 170, a product of the ABCB1 gene. In children, some severe seizure syndromes associated with progressive brain damage are very difficult to treat. In adults, some focal seizures are refractory to medications. Some, particularly in the temporal lobe, are amenable to surgical resection. Some of the drug-resistant population may respond to vagus-nerve stimulation (VNS), a nonpharmacologic treatment for epilepsy now widely approved for treatment of patients with partial seizures. VNS is indicated for refractory cases or for patients in whom antiseizure drugs are poorly tolerated. Stimulating electrodes are implanted in the left vagus nerve, and the pacemaker is implanted in the chest wall or axilla. Use of this device may permit seizure control with lower doses of drugs. New antiseizure drugs are being sought not only by the screening tests noted above but also by more rational approaches. Compounds are sought that act by one of three mechanisms: (1) enhancement of GABAergic (inhibitory) transmission, (2) diminution of excitatory (usually glutamatergic) transmission, or (3) modification of ionic conductances. I. BASIC PHARMACOLOGY OF ANTISEIZURE DRUGS Chemistry Until 1990, approximately 16 antiseizure drugs were available, and 13 of them can be classified into five very similar chemical groups: barbiturates, hydantoins, oxazolidinediones, succinimides, and acetylureas. These groups have in common a similar heterocyclic ring structure with a variety of substituents (Figure 24-1). For drugs with this basic structure, the substituents on the heterocyclic ring determine the pharmacologic class, either anti-MES or antipentylenetetrazol. Very small changes in structure can dramatically alter the mechanism of action and clinical properties of the compound. The remaining drugs¾carbamazepine, valproic acid, and the benzodiazepines¾are structurally dissimilar, as are the newer compounds marketed since 1990, ie, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, vigabatrin, and zonisamide

R 。C=0 0=C 3N一 Figure 24-1.Antiseizure heterocyclic ring structure.The "X"varies as follows: hydantoin derivatives,-N-;barbiturates,-C-N-;oxazolidinediones,-O-;succinimides, -C-;acetylureas,-NH2(N connected to C2).RI,R2,and R3 vary within each subgroup. Pharmacokinetics The antiseizure drugs exhibit many similar pharmacokinetic properties%even those whose structural and chemical properties are quite diverse%because most have been selected for oral activity and all must enter the central nervous system.Although many of these compounds are only slightly soluble,absorption is usually good,with 80-100%of the dose reaching the circulation.Most antiseizure drugs are not highly bound to plasma proteins. Antiseizure drugs are cleared chiefly by hepatic mechanisms,although they have low extraction ratios.Many are converted to active metabolites that are also cleared by the liver.These drugs are predominantly distributed into total body water.Plasma clearance is relatively slow;many anticonvulsants are therefore considered to be medium-to long-acting.Some have half-lives longer than 12 hours.Many of the older antiseizure drugs are potent inducers of hepatic microsomal enzyme activity. DRUGS USED IN PARTIAL SEIZURES GENERALIZED TONIC-CLONIC SEIZURES INTRODUCTION The classic major drugs for partial and generalized tonic-clonic seizures are phenytoin (and congeners),carbamazepine,valproate,and the barbiturates.However,the availability of newer drugs%lamotrigine,levetiracetam,gabapentin,oxcarbazepine, pregabalin,topiramate,vigabatrin,and zonisamide is altering clinical practice in countries where these compounds are available. PHENYTOIN Introduction Phenytoin is the oldest nonsedative antiseizure drug,introduced in 1938,after a systematic evaluation of compounds such as phenobarbital that altered electrically induced seizures in laboratory animals.It was known for decades as diphenylhydantoin
Figure 24-1. Antiseizure heterocyclic ring structure. The "X" varies as follows: hydantoin derivatives, -N-; barbiturates, -C-N-; oxazolidinediones, -O-; succinimides, -C-; acetylureas, -NH2 (N connected to C2). R1, R2, and R3 vary within each subgroup. Pharmacokinetics The antiseizure drugs exhibit many similar pharmacokinetic properties¾even those whose structural and chemical properties are quite diverse¾because most have been selected for oral activity and all must enter the central nervous system. Although many of these compounds are only slightly soluble, absorption is usually good, with 80-100% of the dose reaching the circulation. Most antiseizure drugs are not highly bound to plasma proteins. Antiseizure drugs are cleared chiefly by hepatic mechanisms, although they have low extraction ratios.Many are converted to active metabolites that are also cleared by the liver. These drugs are predominantly distributed into total body water. Plasma clearance is relatively slow; many anticonvulsants are therefore considered to be medium- to long-acting. Some have half-lives longer than 12 hours. Many of the older antiseizure drugs are potent inducers of hepatic microsomal enzyme activity. DRUGS USED IN PARTIAL SEIZURES & GENERALIZED TONIC-CLONIC SEIZURES INTRODUCTION The classic major drugs for partial and generalized tonic-clonic seizures are phenytoin (and congeners), carbamazepine, valproate, and the barbiturates. However, the availability of newer drugs¾lamotrigine, levetiracetam, gabapentin, oxcarbazepine, pregabalin, topiramate, vigabatrin, and zonisamide is altering clinical practice in countries where these compounds are available. PHENYTOIN Introduction Phenytoin is the oldest nonsedative antiseizure drug, introduced in 1938, after a systematic evaluation of compounds such as phenobarbital that altered electrically induced seizures in laboratory animals. It was known for decades as diphenylhydantoin
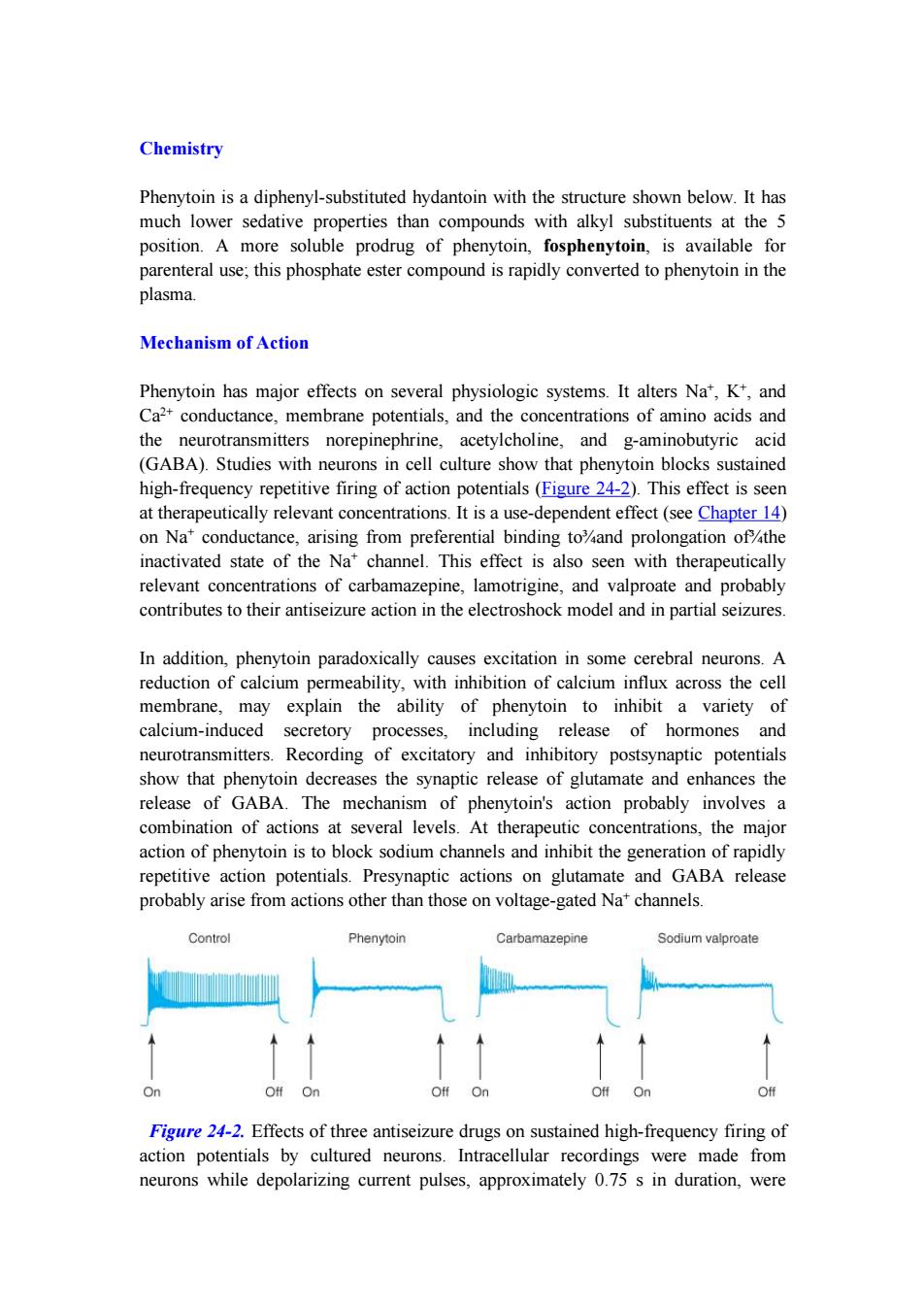
Chemistry Phenytoin is a diphenyl-substituted hydantoin with the structure shown below.It has much lower sedative properties than compounds with alkyl substituents at the 5 position.A more soluble prodrug of phenytoin,fosphenytoin,is available for parenteral use;this phosphate ester compound is rapidly converted to phenytoin in the plasma. Mechanism of Action Phenytoin has major effects on several physiologic systems.It alters Na',K+,and Ca2+conductance,membrane potentials,and the concentrations of amino acids and the neurotransmitters norepinephrine,acetylcholine,and g-aminobutyric acid (GABA).Studies with neurons in cell culture show that phenytoin blocks sustained high-frequency repetitive firing of action potentials(Figure 24-2).This effect is seen at therapeutically relevant concentrations.It is a use-dependent effect(see Chapter 14) on Na conductance,arising from preferential binding to%and prolongation of the inactivated state of the Na'channel.This effect is also seen with therapeutically relevant concentrations of carbamazepine,lamotrigine,and valproate and probably contributes to their antiseizure action in the electroshock model and in partial seizures. In addition,phenytoin paradoxically causes excitation in some cerebral neurons.A reduction of calcium permeability,with inhibition of calcium influx across the cell membrane,may explain the ability of phenytoin to inhibit a variety of calcium-induced secretory processes,including release of hormones and neurotransmitters.Recording of excitatory and inhibitory postsynaptic potentials show that phenytoin decreases the synaptic release of glutamate and enhances the release of GABA.The mechanism of phenytoin's action probably involves a combination of actions at several levels.At therapeutic concentrations,the major action of phenytoin is to block sodium channels and inhibit the generation of rapidly repetitive action potentials.Presynaptic actions on glutamate and GABA release probably arise from actions other than those on voltage-gated Na'channels. Control Phenytoin Carbamazepine Sodium valproate On Off On Off On Off On Off Figure 24-2.Effects of three antiseizure drugs on sustained high-frequency firing of action potentials by cultured neurons.Intracellular recordings were made from neurons while depolarizing current pulses,approximately 0.75 s in duration,were
Chemistry Phenytoin is a diphenyl-substituted hydantoin with the structure shown below. It has much lower sedative properties than compounds with alkyl substituents at the 5 position. A more soluble prodrug of phenytoin, fosphenytoin, is available for parenteral use; this phosphate ester compound is rapidly converted to phenytoin in the plasma. Mechanism of Action Phenytoin has major effects on several physiologic systems. It alters Na + , K+ , and Ca 2+ conductance, membrane potentials, and the concentrations of amino acids and the neurotransmitters norepinephrine, acetylcholine, and g-aminobutyric acid (GABA). Studies with neurons in cell culture show that phenytoin blocks sustained high-frequency repetitive firing of action potentials (Figure 24-2). This effect is seen at therapeutically relevant concentrations. It is a use-dependent effect (see Chapter 14) on Na + conductance, arising from preferential binding to¾and prolongation of¾the inactivated state of the Na + channel. This effect is also seen with therapeutically relevant concentrations of carbamazepine, lamotrigine, and valproate and probably contributes to their antiseizure action in the electroshock model and in partial seizures. In addition, phenytoin paradoxically causes excitation in some cerebral neurons. A reduction of calcium permeability, with inhibition of calcium influx across the cell membrane, may explain the ability of phenytoin to inhibit a variety of calcium-induced secretory processes, including release of hormones and neurotransmitters. Recording of excitatory and inhibitory postsynaptic potentials show that phenytoin decreases the synaptic release of glutamate and enhances the release of GABA. The mechanism of phenytoin's action probably involves a combination of actions at several levels. At therapeutic concentrations, the major action of phenytoin is to block sodium channels and inhibit the generation of rapidly repetitive action potentials. Presynaptic actions on glutamate and GABA release probably arise from actions other than those on voltage-gated Na + channels. Figure 24-2. Effects of three antiseizure drugs on sustained high-frequency firing of action potentials by cultured neurons. Intracellular recordings were made from neurons while depolarizing current pulses, approximately 0.75 s in duration, were

applied (on-off step changes indicated by arrows).In the absence of drug,a series of high-frequency repetitive action potentials filled the entire duration of the current pulse.Phenytoin,carbamazepine,and sodium valproate all markedly reduced the number of action potentials elicited by the current pulses.(Modified and reproduced, with permission,from Macdonald RL,Meldrum BS:Principles of antiepileptic drug action.In:Levy RH,et al [editors]:Antiepileptic Drugs,4th ed.Raven Press,1995.) Clinical Use Phenytoin is effective against partial seizures and generalized tonic-clonic seizures.In the latter,it appears to be effective against attacks that are either primary or secondary to another seizure type. Pharmacokinetics Absorption of phenytoin is highly dependent on the formulation of the dosage form. Particle size and pharmaceutical additives affect both the rate and the extent of absorption.Absorption of phenytoin sodium from the gastrointestinal tract is nearly complete in most patients,although the time to peak may range from 3 hours to 12 hours.Absorption after intramuscular injection is unpredictable,and some drug precipitation in the muscle occurs;this route of administration is not recommended for phenytoin.In contrast,fosphenytoin,a more soluble phosphate prodrug of phenytoin,is well absorbed after intramuscular administration. Phenytoin is highly bound to plasma proteins.The total plasma level decreases when the percentage that is bound decreases,as in uremia or hypoalbuminemia,but correlation of free levels with clinical states remains uncertain.Drug concentration in cerebrospinal fluid is proportionate to the free plasma level.Phenytoin accumulates in brain,liver,muscle,and fat. Phenytoin is metabolized to inactive metabolites that are excreted in the urine.Only a very small proportion of phenytoin is excreted unchanged. The elimination of phenytoin is dose-dependent.At very low blood levels,phenytoin metabolism follows first-order kinetics.However,as blood levels rise within the therapeutic range,the maximum capacity of the liver to metabolize phenytoin is approached(Figure 24-3).Further increases in dosage,even though relatively small, may produce very large changes in phenytoin concentrations.In such cases,the half-life of the drug increases markedly,steady state is not achieved in routine fashion (since the plasma level continues to rise),and patients quickly develop symptoms of toxicity. The half-life of phenytoin varies from 12 hours to 36 hours,with an average of 24 hours for most patients in the low to mid therapeutic range.Much longer half-lives are observed at higher concentrations.At low blood levels,it takes 5-7 days to reach
applied (on-off step changes indicated by arrows). In the absence of drug, a series of high-frequency repetitive action potentials filled the entire duration of the current pulse. Phenytoin, carbamazepine, and sodium valproate all markedly reduced the number of action potentials elicited by the current pulses. (Modified and reproduced, with permission, from Macdonald RL, Meldrum BS: Principles of antiepileptic drug action. In: Levy RH, et al [editors]: Antiepileptic Drugs, 4th ed. Raven Press, 1995.) Clinical Use Phenytoin is effective against partial seizures and generalized tonic-clonic seizures. In the latter, it appears to be effective against attacks that are either primary or secondary to another seizure type. Pharmacokinetics Absorption of phenytoin is highly dependent on the formulation of the dosage form. Particle size and pharmaceutical additives affect both the rate and the extent of absorption. Absorption of phenytoin sodium from the gastrointestinal tract is nearly complete in most patients, although the time to peak may range from 3 hours to 12 hours. Absorption after intramuscular injection is unpredictable, and some drug precipitation in the muscle occurs; this route of administration is not recommended for phenytoin. In contrast, fosphenytoin, a more soluble phosphate prodrug of phenytoin, is well absorbed after intramuscular administration. Phenytoin is highly bound to plasma proteins. The total plasma level decreases when the percentage that is bound decreases, as in uremia or hypoalbuminemia, but correlation of free levels with clinical states remains uncertain. Drug concentration in cerebrospinal fluid is proportionate to the free plasma level. Phenytoin accumulates in brain, liver, muscle, and fat. Phenytoin is metabolized to inactive metabolites that are excreted in the urine. Only a very small proportion of phenytoin is excreted unchanged. The elimination of phenytoin is dose-dependent. At very low blood levels, phenytoin metabolism follows first-order kinetics. However, as blood levels rise within the therapeutic range, the maximum capacity of the liver to metabolize phenytoin is approached (Figure 24-3). Further increases in dosage, even though relatively small, may produce very large changes in phenytoin concentrations. In such cases, the half-life of the drug increases markedly, steady state is not achieved in routine fashion (since the plasma level continues to rise), and patients quickly develop symptoms of toxicity. The half-life of phenytoin varies from 12 hours to 36 hours, with an average of 24 hours for most patients in the low to mid therapeutic range. Much longer half-lives are observed at higher concentrations. At low blood levels, it takes 5-7 days to reach