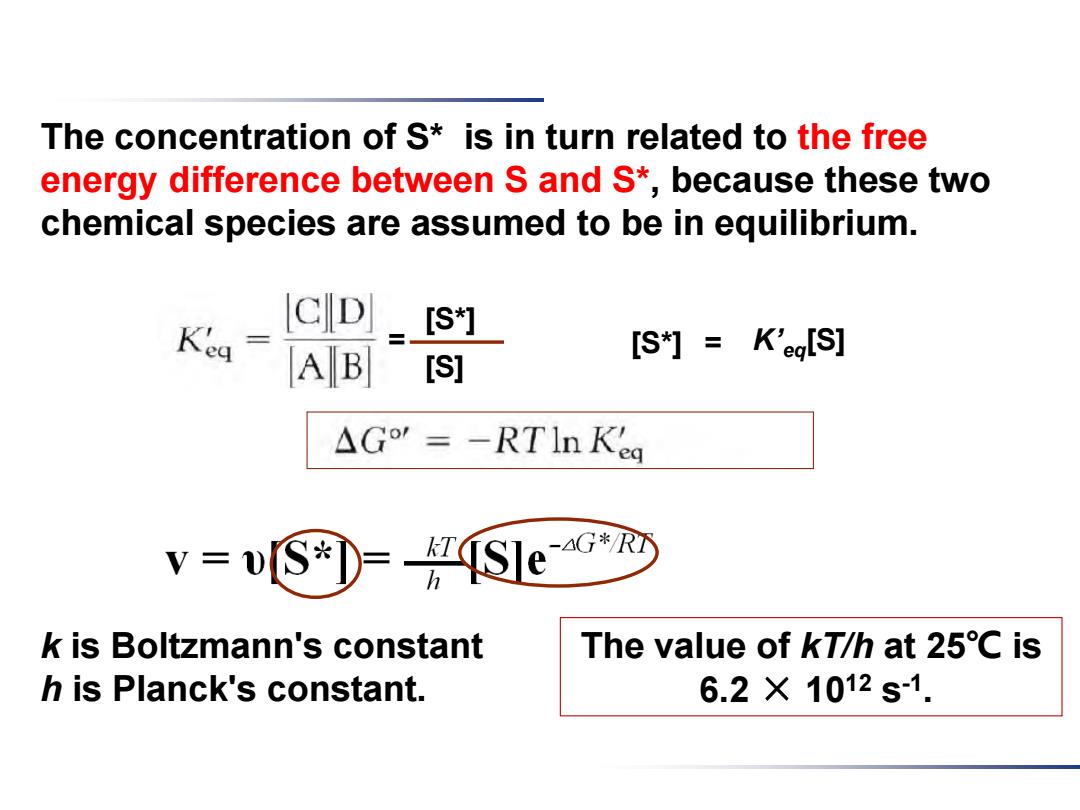
The concentration of S*is in turn related to the free energy difference between S and S*,because these two chemical species are assumed to be in equilibrium. CD [S*灯] A B [S*灯=K'egIS] [S] △G'=-RTIn Kea V -[S)=S]e k is Boltzmann's constant The value of kT/h at 25C is h is Planck's constant. 6.2×1012s-1
The concentration of S* is in turn related to the free energy difference between S and S*, because these two chemical species are assumed to be in equilibrium. k is Boltzmann's constant h is Planck's constant. = [S*] [S] [S*] = K’eq[S] The value of kT/h at 25℃ is 6.2 × 1012 s -1
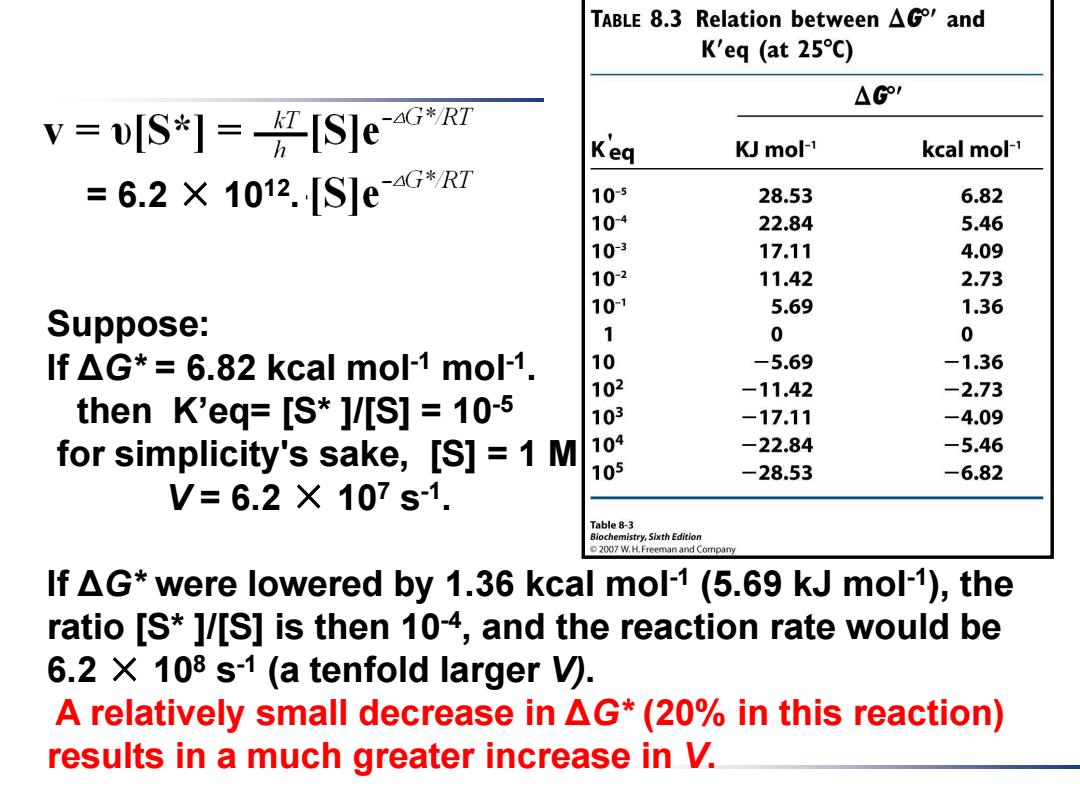
TABLE 8.3 Relation between AG and K'eq (at 25C) △G1 v=wlS*1=任SleG Keq KJ mol-1 kcal mol-1 =6.2×1012.[S]eG*R7 105 28.53 6.82 104 22.84 5.46 10-3 17.11 4.09 10-2 11.42 2.73 10-1 5.69 1.36 Suppose: 1 0 0 If AG*=6.82 kcal mol-1 mol-1. 10 -5.69 -1.36 102 -11.42 -2.73 then K'eq=[S*]/[S]=10-5 103 -17.11 -4.09 for simplicity's sake,[S]=1 M 104 -22.84 -5.46 105 -28.53 -6.82 V=6.2×107s1. Table 8-3 Biochemistry,Sixth Edition 2007 W.H.Freeman and Company If AG*were lowered by 1.36 kcal mol-1(5.69 kJ mol-1),the ratio [S*]/[S]is then 104,and the reaction rate would be 6.2 X 108 s-1(a tenfold larger V). A relatively small decrease in AG*(20%in this reaction) results in a much greater increase in V
= 6.2 × 1012 . Suppose: If ΔG* = 6.82 kcal mol-1 mol-1 . then K’eq= [S* ]/[S] = 10-5 for simplicity's sake, [S] = 1 M V = 6.2 × 107 s -1 . If ΔG* were lowered by 1.36 kcal mol-1 (5.69 kJ mol-1 ), the ratio [S* ]/[S] is then 10-4 , and the reaction rate would be 6.2 × 108 s -1 (a tenfold larger V). A relatively small decrease in ΔG* (20% in this reaction) results in a much greater increase in V