
protein can be scen.Estimate their percentages can be performed by spectrophotometer scan. Procedure: 1.Preparation Suitable volume electrophoresis buffer is pour into electrophoresis bath and keep the surface of the buffer in two cells through a inter connector.Four layers of bandage are put on two inter supporting board to put up a bridge that connect,with one edge of the bandage on the supporting board and the opposite edge soaked in the buffer. 2.Sample loading Immerse an 8cm x 2cm cellulose acetate membrane into the electrophoresis buffer for several hours.take it out and absorb water on the membrane with filter paper.Draw a marker line that parallel and with 2 cm distance to one end using pencil. This is the sample loading line Put the non-lustrous side of the cellulose acetate membrane upward.To moisten a little sample on one edge of a glass slide and then put this edge onto the sample line. Let the sample be absorbed into the membrane.Be careful to load the sample on a well-distributedine. 3.Electrophoresis Put the membrane with sample on the electrophoresis device,with the sample line end near to anode,and with the non-lustrous side of the membrane downward well-settledt the bandage and keep the membranc Examine the connection of the anode and cathode.Adjust the voltage to 110V. electrophoresis for 45 to 60 minutes. 4.Staining Turn off the power and take the membrane out.Soaking the membrane in staining solution for five minutes.Then rinse the membrane in rinsing solution for several times,until the background of the membrane become white. 5.Quantitative analysis Observe and judge the shade of color and width of the bands by naked eve to estimate their quantity

Cut each band along the e of band shade and a cossate membrane which width e san e as the proteir band.Put albumin band int tube containing 6ml of 0.4mol/L NaOH and rest of them in to 3ml of 0.4molL NaOH respectively.Shake tubes constantly.Or put them in 37 C water bath if necessary. After complete decoloration,adjust zero with control tube,the light absorption of each tubes are measured at 620nm by using 722 spectrophotometry. Additional information: 1.Some parameters of serum proteins serum protein MW 16 69000 alphal-globin 5.06 200000 2 alpha2-globin 5.06 300000 4-9 beta-globins 5.12 90000150000 6.512 gamma-globins6.85~7.3156000~950000 12-20 2.Clinical application For example,in cirrhosis of liver,the concentration of albumin decreases significantly whereas gamma-globins increases 2-3 folds;nephrosis syndrome and chronic glomerulonephritis shows a decrease in albumin and an increase in alpha2- and beta globins;in the of can he between beta-and gamma-globins;alpha fetoprotein is found between alphal-globin and albumin in primarily liver canner. Questions: 1.protein moves faster than others?What is the potein that corresponding to each band on the membrane?Why? 2.Which electrode should be near to the end of the membrane that containing sample line?Why? Reagents: 1.The electrophoresis buffer:barbital sodium-barbital(pH8.6,ion intensity 0.06) Take 12.36 g barbital sodium and 1.66 g barbital and dissolve them in 1000 ml distilled water. 2.Amino black 10B dye solution:0 5 g amino black 10B is dissolved in 50 ml methanol and then 10 ml acetic acid and 40 ml water is added,mix adequately 3.Rinsing solution:45 ml methanol and 5 ml acetic acid are dissolved in 50 ml water, mix adequately

Experiment 4 Extraction and Purification of Alkline Phosphotase Extraction and purification of enzymes are one basic technology.Up to now most of enz mes found are the similar mthods to extract and purify them like alkaline phosphatase(AKP). Purpose Master the principle and method of enzyme purification and extraction by organic solven Principle In this study,we intend to use organic solvent precipitation method to extract AKP from liver mogenate.Enz uld be de y ethona acetol,n-bulanol and ete.Organic solvent could 1 mak ke enzyme dehydration anc dielectric constant decline.Because of declination of dielectric constant.the affinity of proteins carrying opposite charge increases,resulting in enzyme protein agglutination from solvent.However,this sort of organic solvent could dissolve in water,and so make more proteins separation from solvent. Low concentration natrium aceticum is used in the preparation of liver homogenate.in order to permeabilize cell membrane.while mgac could protect and maintain AKP.AKP is denaturated by n-bulanol,filtered,and extracted and purified by ice ethonal and acetol.AKP could dissolve in 33%acetol or 30%ethonal,yet could not dissolve in50%% onal of fina volume we can use repeated centrifugation to get part purified AKP Note that many organic solvent could make enzymes disabled,so the purification process should exert under 0C.Organic solvent should be precooled,dropped slowly and mixed well to prevent enzyme ation from over too hot in part.Separated nzymes arecasily precipitated by concentrifugation It is better to dissolve the extract in proper cold water or buffer in order to keep enzyme activity.lonic strength is another point other than PH and enzyme concentration. Protocol 1.Cut fresh cony liver 2.5 g into pieces,and put it into a glass homogenate tube. then add 2.5 ml 0.01 M MgAc2-0.01 M NaAc solvent into the tube,grind

sufficiently.Transfer the homogenate into a graduated centrifuge tube.and wash the homogenate tube with total volume of 5 ml solvent.Mix the wash and well,and mark it as solution A with 10ml total volume.Pipe 0.2 ml solvent A into another tube which is tagged as A. 2.Add 2.5 ml n-bulanol into the residual solvent,mix completely for 2 minutes tube 3.Add isovolumic volume ice acetol and mix well at once.Centrifuge the solvent for 5 minutes at 3000 rpm.Discard the supernatant,and add 4 ml 0.5 M MgAc2. Agitate it completely in order to dissolve it with a glass rod,meanwhile record the volume.This is solution B.Pipe 0.2 ml solvent A into another tube which is tagged as B. 4.Record the volume of the residual suspension,and add 96%ethanol to make the final concentration of ethanol to 30%.(Details refer to appendix).Shake up and record the volume. Add 96% ethano make the final concentration of ethanol to60%(sce appendix).Mix completely and centrifuge for 5 minutes at 3000 rpm.Discard the supernatant,and add 4 ml 0.5 M MgAcz.Agitate it completely in order to make the precipitation dissolved. 5. Add ice aceto nt the solutio om step 4.and mak e the final co of acetone to 33% Mix completely and centrifuge (3000 rpm)for 5 minutes Transfer the supernatant to another tube and record the volume.Add ice acetone slowly to make the final concentration to 50%.Mix completely and centrifuge for 5 minutes at 3000 rpm.The precipitation is partly purified AKP.Add 4 ml Tris Buffer(pH 8.8)to dissolve the eprecipitation.This is solution C. 6.Sample dilution:dilute samples as following to determine the AKP activity and concentration. Solution A A samples(ml) 02 0.2 0.5 Tris Buffer(ml) 3.8 18 I5 Post-dilution A20 B Dilution factor 10 Note:A-A,diluted 80 folds
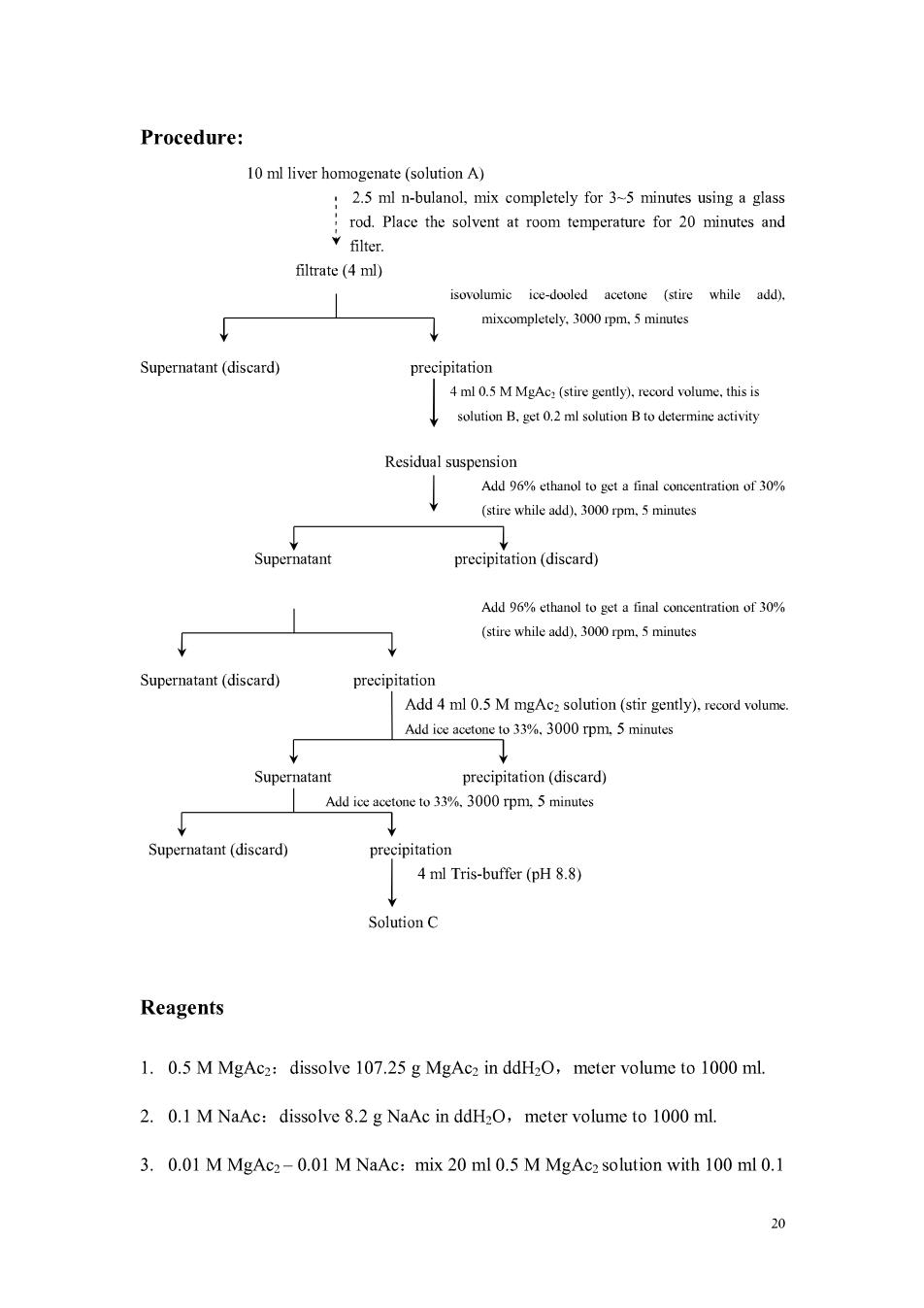
Procedure: :2.5 ml n-bulanol,mix completely for 3-5 minutes using a glass rod.Place the solvent at room temperature for 20 minutes and filter. filtrate(4 ml) ice-dooled aceton (stire whilea) mixcompletely0m5minutes Supernatant(discard) precipitation 4 ml 0.5 M MeAc,(stire gently).record volume.this is Residual suspension Add 96%ethanol to get a final concentration of 30% (stire while add).3000 rom.5 minutes atant Add%final of (stire while add).3000 rpm.5 minutes Supernatant(diseard) Precipitation Add 4ml0.5 MmgAc:solution (stir gently).e Add ice acetone to 33%.3000 rpm.5 minutes Supe atant precipitation(diseard) Add ice acetone to 33%.3000 rpm.5 minutes precipitation 4 ml Tris-buffer(pH 8.8) SolutionC Reagents 1.0.5 M MgAc2:dissolve 107.25 g MgAcz in ddH2O,meter volume to 1000 ml. 2.0.1 M NaAc:dissolve 8.2 g NaAc in ddH2O,meter volume to 1000 ml. 3.0.01 M MgAc2-0.01 M NaAc:mix 20 ml 0.5 M MgAc2 solution with 100 ml 0.1