
Biochemistry Experiment Direction TaiShan Medical College

Contents N0ti沁.4 Experimental Report 4,4 PART I:experiments PROTEINS 1.Protein concentration determination.5 Protein Determination by UV Absorption The Lowry Method for Protein Quantitation The Bicinchoninic Acid(BCA)Assay for Protein Quantitation 2.Purification of Y-globulin from human serum.14 3.Cellulose acetate membrane electrophoresis of serum protein.16 ENZYME 4.Extraction and Purification of Alkline 5.Specific Activity of Alkline Phosphatase. .23 6.Enzyme Kinetics:Substrate Concentration Affecting Enzyme Activity -Michaelis Constant Assay of Alkline Phosohatase. .28 NUTRITION METABOLISM 7.The measurement of the blood sugar and effects of adrenalin on blood sugar concentration.32 8.Determinations of Total Serum Triglyceride 35 9.Identification of the transamination by paper chromatography.37 10.Determination of arginase activity of the liver .40 MOLECULAR BIOCHEMISTRY 11.Isolation and Purification of Nucleic Acid44 12.Agarose Gel Electrophoresis of DNA 46 PART II:Principles of Biochemistry experiments Chap.1,Basic Theories of biochemistry experiment.49 Chap.2.Spectrophotometry .58 Chap.3.Electrophoresis .60

Notice 1.Do preparation on subject matter,principles and protocol of the experiment before going to the laboratory: 2.Operate accurately,observe carefully,record veraciously during the experiment; 3,Accomplish report and clean the bench after the experiment: 4.Obey the disciplines of the lab,keep safety and take care of instruments; 5.Ware experimental overcoat,do not ware sandal,bring this booklet and your repor notebook with you; 6.Check the quality and quantity of your experiment suite before and after the experiment and tell the technician if there is any mar; 7.Clean your xperimental suite and discard garbage,especially dangerous castoff. under the teacher's guidance; 8.Clean the laboratory when you are on the duty of the day. How to write experimental record and experimental report Experimental report should include five parts,purpose,principle,operation. esu and disc sion Before ach purpose,principle and operation of the experiment briefly on your report notebook The record during the experiment should be veracious and timely,which includes reagents (volume,concentration etc.),conditions (temperature,pressure etc.). instruments(name,type etc.),operations(shake,churn up etc.)and phenomena(color precipitation, lata on the indic r and so on).Data processing ar nd analyzing and result summarizing should be done in the result part of the experimenta report. discussion part,you might want to focus on the analysis of experimental methods, results phenomena and errors,and your taste and comments. Experimental record and report should be clear,logistic,concise and no disorder alteration. 3

PART I:EXPERIMENTS Experiment 1 Protein concentration determination According to the proteins'physical,chemical or biological properties,we have developed lots of methods to determining protein concentration.In this article we will study the principle of several protein determination methods to understand their protocols and application. The Lowry method for protein quantitation 1.Principle td is based on both the Biuret reaction.n which the peptide bonds of to prod which 11B0010-u10.J111p with coppe reacts but in essence phosphomolybdotungstate is reduced to heteropolymolybdenum blue by the copper-catalyzed oxidation of aromatic amino acids.The reactions result in a strong blue color,which depends partly on the tyrosine and tryptophan content. Sensitivity of the procedure of Lowry et al.is moderately constant from protein to protein,and it has been so widely used that Lowry protein estimations are a completely acceptable alternative to a rigorous absolute determination in almost all circumstances in which protein mixtures or crude extracts are involved.The method is sensitive down to about 0.01 mg of protein/mL,and is best used on solutions with concentrations in the range 0.01-1.0 mg/mL of protein 2.Materials 1.Standards:Use a stock solution of standard protein (e.g.bovine serum albumin fraction V)containing 250 ug/mL protein in distilled water,stored frozen at _20℃ 2.Complex-forming reagent:Prepare immediately before use by mixing the following stock solutions in the proportion 50:50:1:1 (by vol).(first mix A and B.C and D,respectively,and then mix the two solutions): Solution A:4%(w/v)NazCO;in distilled water Solution B:0.2 mol/L NaOH in distilled water olutio C. 1%(w/v)CuSO5H2O in distilled water Solution D:2%(w/v)sodium potassium tartrate in distilled water. 3.Folin reagent(commercially available):Use at 1 M concentration. 4.Sample:human serum diluted 100 folds with distilled water,store frozen at -20℃. 4
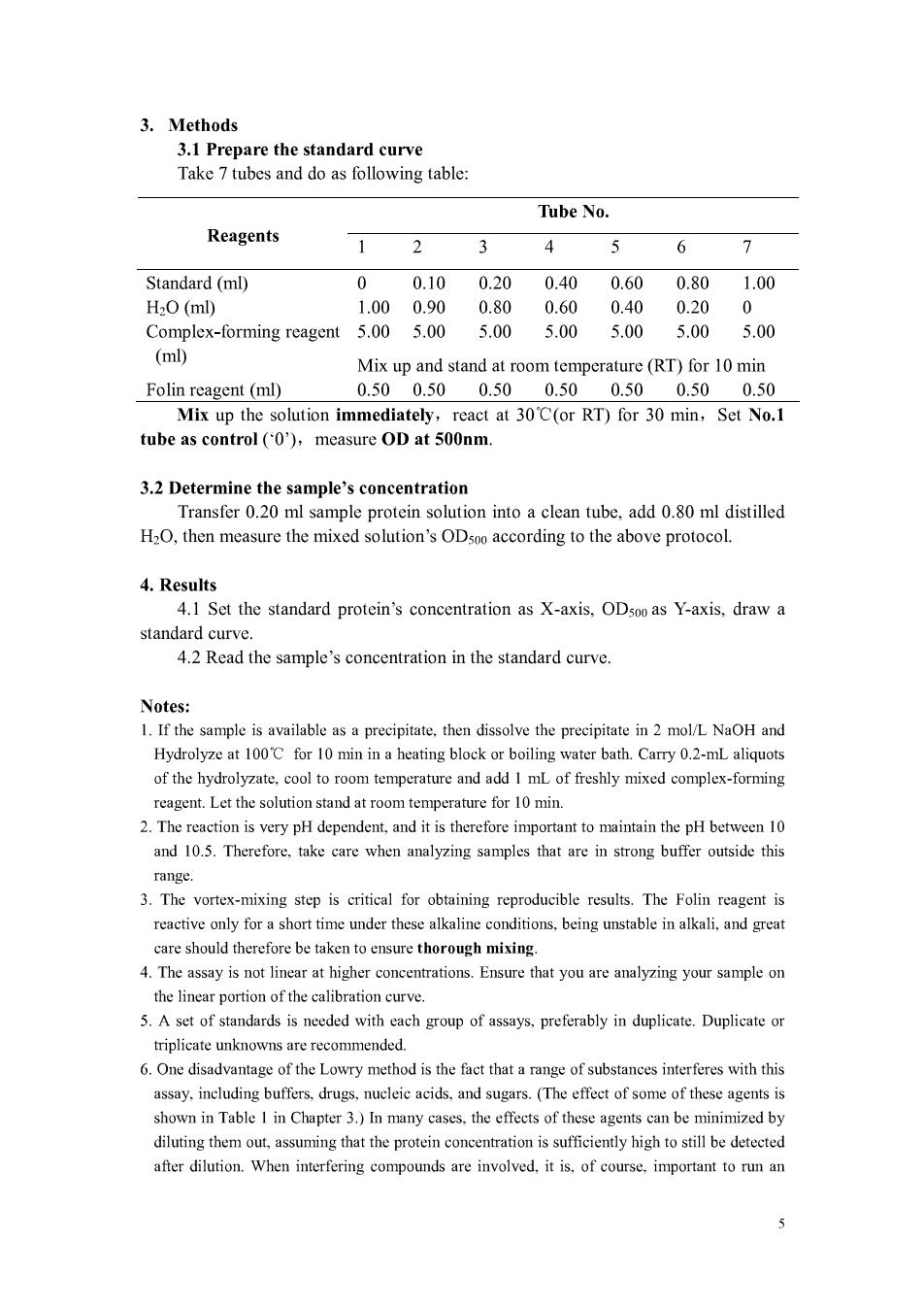
3.Methods 3.1 Prepare the standard curve Take 7 tubes and do as following table: Tube No. Reagents 1 2 3 4 5 6 7 Standard(ml) 010 020 040060 0.801.00 H2O(ml) 1.000.90 0.80 0.60 0.40 0.20 Complex-forming reagent 5.00 5.00 5.00 5.00 5.00 5.00 5.00 (ml) Mix up and stand at room temperature(RT)for 10 min Folin reagent(ml) 0.500.500.500.500.500.500.50 Mix up the solution immediately,react at 30'C(or RT)for 30 min,Set No.1 tube as control(0) measure OD at 500nm 3.2 Determine the sample's concentration Transfer 0.20 ml sample protein solution into a clean tube,add 0.80 ml distilled H2O,then measure the mixed solution's ODsoo according to the above protocol. 4.Results 4.1 Set the standard protein's concentration as X-axis.OD500 as Y-axis.draw a standard curve. 4.2 Read the sample's concentration in the standard curve savailable as a precipitate,then dissolve the precipitate in 2 Hydrolyze at 100'C for 10 min in a heating block or boiling water bath.Carry 0.2-mL aliquots of the hydrolyzate.cool to room temperature and add I mL of freshly mixed complex-forming reagent.Let the solution stand at room tem perature for 10min. 2.The reaction is v pH dependent,and it therefor mportant to maintain the pH between10 and 10.5.Therefore.take are when analyzing sa that are in stronbuffer outside this range 3.The vortex-mixing step is critical for obtaining reproducible results.The Folin reagent is reactive only for a short time under these alkaline conditions,being unstable in alkali,and great care should therefore be taken thoroug mixing. 4.The assay is not line at higher ion.Ensure that you are your sampon the linear portion of the calibration curve. 5.A set of standards is needed with each group of assays,preferably in duplicate.Duplicate or triplicate unknowns are recommended. 6.One disadvantage of the Lowry method is the fact that a range of substanees interferes with this includin s.drugs, ucleic acids.d sugars.(Th effect of some of these agents is shown in Table I in Chapter 3.)In many cases.the effects of these agents can be minimized by diluting them out,assuming that the protein concentration is sufficiently high to still be detected after dilution.When interfering compounds are involved,it is,of course.important to run an