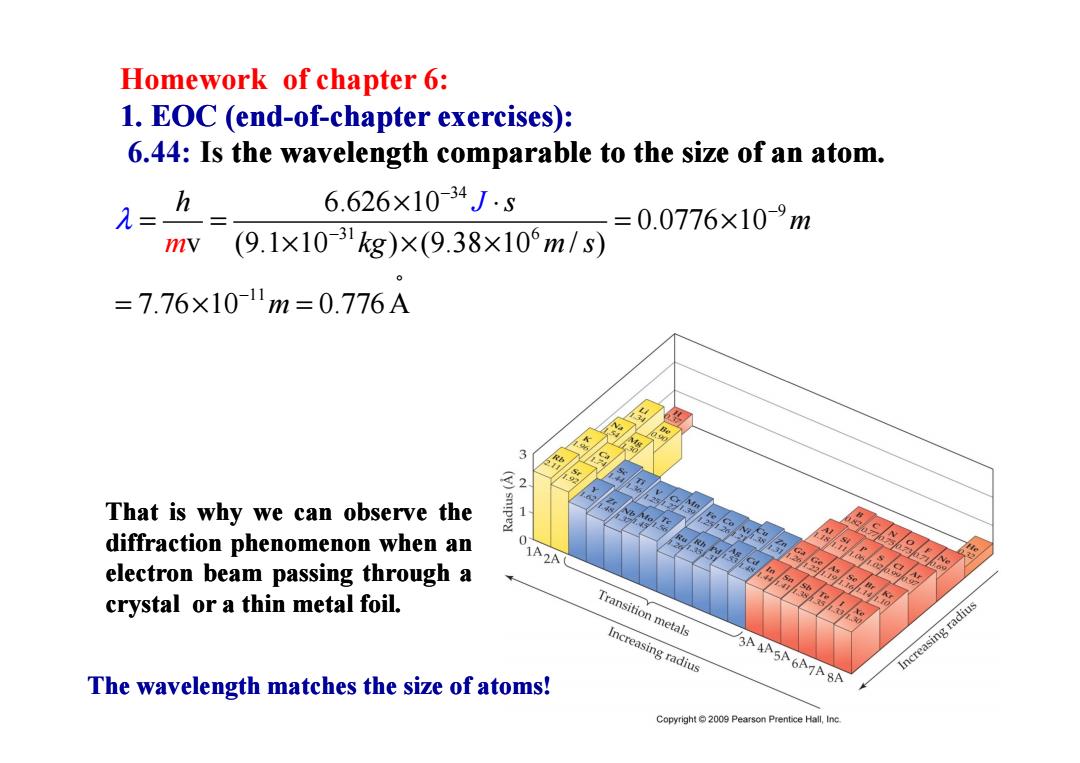
Homework of chapter 6: 1.EOC (end-of-chapter exercises): 6.44:Is the wavelength comparable to the size of an atom. h 6.626×10-34J.s = 0.0776×10-9m mv (9.1×10-31kg)×(9.38×10m/s) =7.76×10-1m=0.776A 32 That is why we can observe the diffraction phenomenon when an 0 bessasshee ® electron beam passing through a C8 crystal or a thin metal foil. Transition metals Increasing radius A AASA6A7A8A Increasing radius The wavelength matches the size of atoms! Copyright 2009 Pearson Prentice Hall,Inc
Homework of chapter 6: 1. EOC (end 1. EOC (end-of-chapter exercises): chapter exercises): 6.44: Is the wavelength comparable to the size of an atom. the wavelength comparable to the size of an atom. 34 9 31 6 11 6.626 10 0.0776 10 v (9.1 10 ) (9.38 10 / ) 7.76 10 0.776 A m h s m kg m s m J λ − − − ° − × ⋅ = = = × × × × = × = That is why we can observe observe the diffraction diffraction phenomenon phenomenon when an electron electron beam passing passing through through a crystal crystal or a thin metal foil. The wavelength matches the size of atoms!
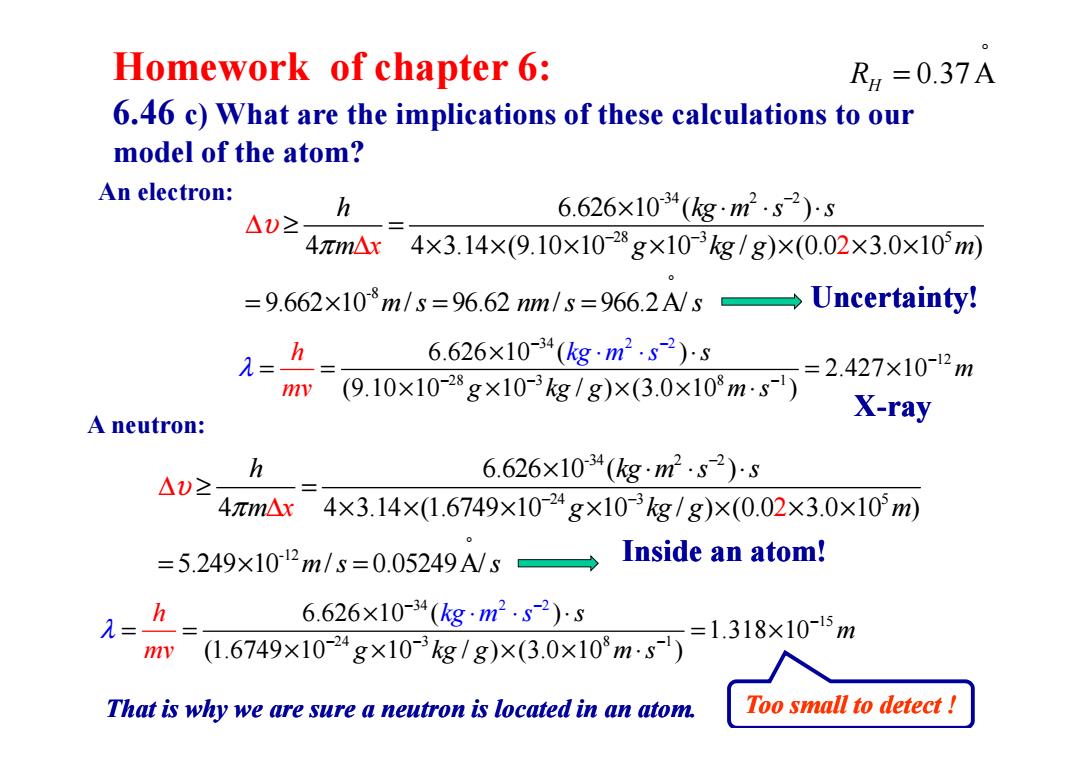
Homework of chapter 6: RH=0.37A 6.46 c)What are the implications of these calculations to our model of the atom? An electron: h 6.626×1034(gm2.s2)s △)2 4元m△x 4×3.14×(9.10×10-28g×103g/g)×(0.02×3.0×10m) =9.662x10m/s=96.62 nm/s=966.2A/sUncertainty! =- h 6.626×10-34(kgm2.s-2)s =2.427×10-12m mv (9.10×10-28g×10-3kg/g)×(3.0×10m·s) X-ray A neutron: h 6.626×1034(gm2.s2)s △U2 4元m△x 4×3.14×1.6749×10-24g×103kg/g)×(0.02×3.0×10m) =5.249×102m/s=0.05249A/5→ Inside an atom! 6.626×10-34(kg·m2.s-2)5 =1.318×10-15m (1.6749×10-24g×10-3kg/g)×(3.0×103m-s) That is why we are sure a neutron is located in an atom. Too small to detect!
34 12 28 2 3 8 2 1 6.626 10 ( ) 2.427 10 (9.10 10 10 / ) (3.0 10 ) s m g kg g m h kg m mv s s λ − − − − − − × ⋅ = = = × × × × × ⋅ ⋅ ⋅ Homework of chapter 6: 6.46 c) What are the implications of these calculations to our model of the atom? -34 2 2 28 3 5 -8 6.626 10 ( ) 4 4 3.14 (9.10 10 10 / ) (0.0 3.0 10 ) 9.662 10 / 96.62 / 966.2A 2 / h kg m s s m g kg g m m s nm s s x υ π − − − ° × ⋅ ⋅ ⋅ ≥ = × × × ∆ ∆ × × × × = × = = An electron: Uncertainty! ° RH = 0.37 A 28 3 8 1 2.427 10 (9.10 10 10 / ) (3.0 10 ) m mv g kg g m s λ − − − = = = × × × × × ⋅ 34 15 24 3 8 1 2 2 6.626 10 ( ) 1.318 10 (1.6749 10 10 / ) (3.0 10 ) s m g kg g m h g m s s mv k λ − − − − − − × ⋅ = = = × ⋅ × × × × ⋅ ⋅ That is why we are sure a neutron is located in an atom. A neutron: -34 2 2 24 3 5 -12 6.626 10 ( ) 4 4 3.14 (1.6749 10 10 / ) (0.0 3.0 10 ) 5.249 10 / 0.05249A/ 2 h kg m s s m g kg g m m s s x υ π − − − ° × ⋅ ⋅ ⋅ ∆ ≥ = ∆ × × × × × × × = × = X-ray Inside an atom! Too small to detect !

Homework of chapter 6: 6.46 c)What are the implications of these calculations to our model of the atom? Nucleus ~10-4 <—1-5A
Homework of chapter 6: 6.46 c) What are the implications of these calculations to our model of the atom? Nucleus
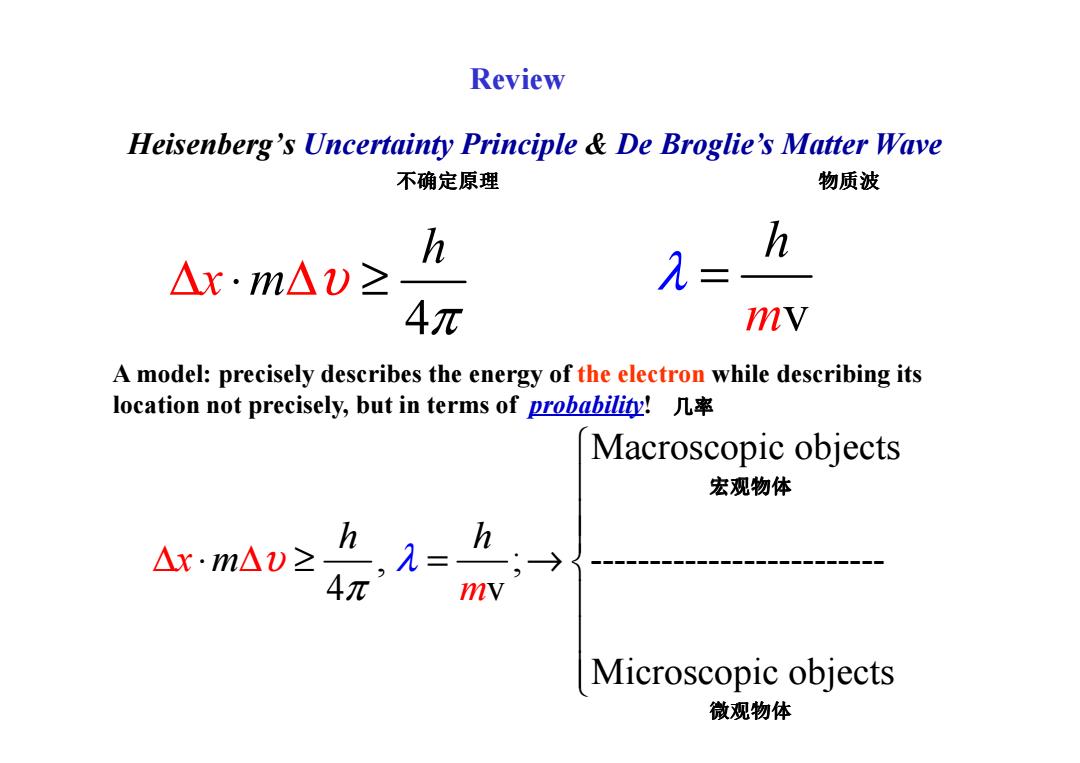
Review Heisenberg's Uncertainty Principle De Broglie's Matter Wave 不确定原理 物质波 h h △x·m△)≥ = 4元 mV A model:precisely describes the energy of the electron while describing its location not precisely,but in terms of probability! Macroscopic objects 宏观物体 h △x·m△v≥ h,= mv Microscopic objects 微观物体
Heisenberg’s Uncertainty Principle & De Broglie’s Matter Wave 4 h x m υ π ∆ ⋅ ∆ ≥ mv h λ = A model: precisely describes the energy of the electron while describing its 不确定原理 物质波 Review Macroscopic objects , ; ------------------------- 4 v Microscopic objects x h m h m υ π λ ∆ ∆ ⋅ ≥ = → A model: precisely describes the energy of the electron while describing its location not precisely, but in terms of probability! 几率 宏观物体 微观物体
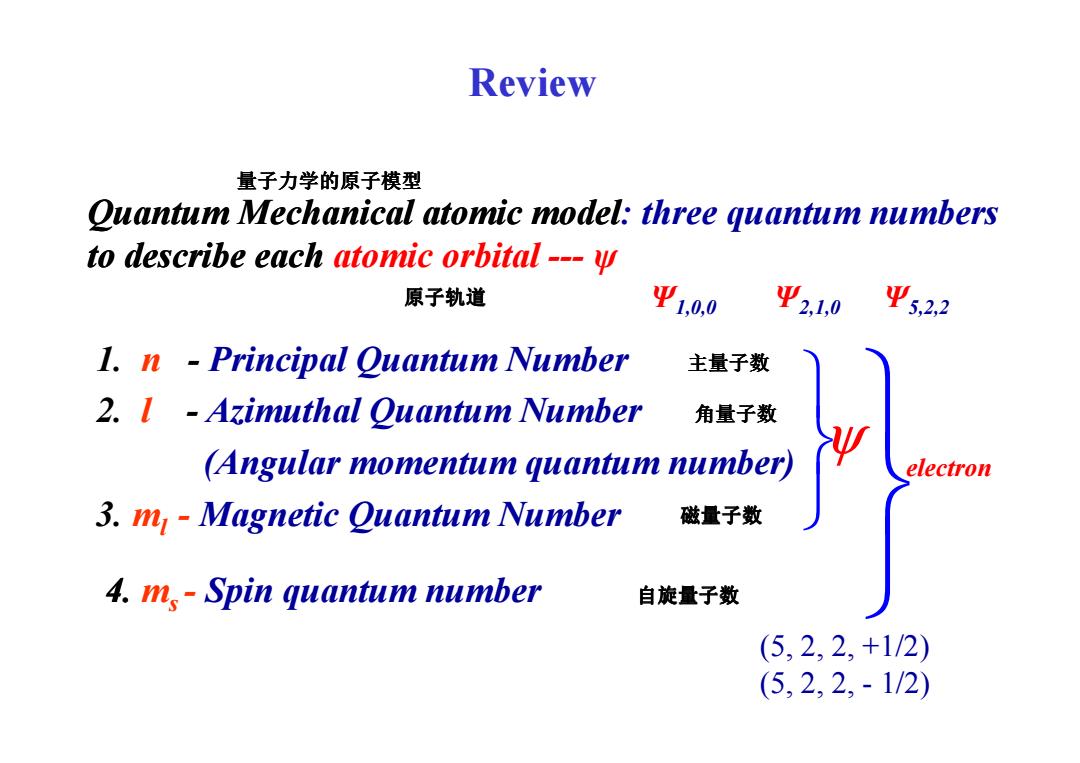
Review 量子力学的原子模型 Quantum Mechanical atomic model:three quantum numbers to describe each atomic orbital---y 原子轨道 Ψ1,0,0 Ψ21,0 Ψ52,2 1.n -Principal Quantum Number 主量子数 2.I Azimuthal Quantum Number 角量子数 (Angular momentum quantum number) electron 3.m Magnetic Quantum Number 磁量子数 4.m,-Spin quantum number 自旋量子数 (5,2,2,+1/2) (5,2,2,-1/2)
Quantum Mechanical atomic model: three quantum numbers to describe each atomic orbital --- ψ 1. n - Principal Quantum Number Review 主量子数 量子力学的原子模型 原子轨道 Ψ1,0,0 Ψ2,1,0 Ψ5,2,2 2. l - Azimuthal Quantum Number (Angular momentum quantum number) 3. ml - Magnetic Quantum Number 4. m s - Spin quantum number ψ 角量子数 磁量子数 自旋量子数 electron (5, 2, 2, +1/2) (5, 2, 2, - 1/2)