
Team work report---"Chemistry at work---Organic Dye" a.What are HUMO and LUMO? b.What are the delocalized n electrons and delocalized n bonds? c.Why some substances,such as organic dyes,are colorful?briefly explain the reasons according to what you have learnt from"Organic dyes"(in the light of molecular orbital theory).What kinds of organic compounds are typical colorful materials? d.Answer the question of 9.93 9.104(f); (a)What is the hybridization at the N atom in each of the substances? (b)How many unhybridized atomic orbitals are there on the N and the C atoms in each ofthe substances? (c)Predict the N-N-C angles in each of the substances. (d)Azobenzene is said to have greater delocalization of its pi electrons than in hydrazobenzene.Discuss this statement. (e)All the atoms of azobenzene lie in one plane,whereas those of the azobenzene do not.Is this observation consistent with the statement in part(a)? (f)Azobenzene is an intensive orange color,whereas hydrazobenzene is nearly colorless.(How many conjugated pi electrons are delocalized in these two compounds?) (f)Benzene,naphthalene and anthracene are colorless,Tetracene is orange,how can this observation be explained? e.List the questions which you didn't understand after your learning and discussion
Team work report ---“Chemistry at work Chemistry at work---Organic Dye Organic Dye” a. What are HUMO and LUMO? b. What are the delocalized b. What are the delocalized π electrons and delocalized electrons and delocalized π bonds? c. Why some substances, such as organic dyes, are colorful? briefly explain the reasons according to what you have learnt from “Organic dyes” ( according to what you have learnt from “Organic dyes” (in the light of molecular orbital theory). What kinds of organic compounds are typical colorful materials? d. Answer the question of 9.93 & 9.104(f); (a)What is the hybridization at the N atom in each of the substances? (b)How many unhybridized atomic orbitals are there on the N and the C atoms in each of the substances? (c) Predict the N (c) Predict the N-N-C angles in each of the substances. C angles in each of the substances. (d) Azobenzene is said to have greater delocalization of its pi electrons than in hydrazobenzene hydrazobenzene. Discuss this statement. . Discuss this statement. (e) All the atoms of azobenzene lie in one plane, whereas those of the azobenzene do not. Is this observation consistent with the statement in part (a)? (f) Azobenzene is an intensive orange color, whereas (f) Azobenzene is an intensive orange color, whereas hydrazobenzene is nearly obenzene is nearly colorless. (How many conjugated pi electrons are delocalized in these two compounds?) (f) Benzene, naphthalene and (f) Benzene, naphthalene and anthracene anthracene are colorless, are colorless, Tetracene Tetracene is orange, how can is orange, how can this observation be explained? e. List the questions which you didn’t understand after your learning and discussion

Questions in your team work reports 1."Why Azobenzene is said to have greater delocalization of its electrons than hydrazobenzene?"We don't know why the n electrons of N should be delocalized?We think the bond between N-N is just a double bond rather than a delocalized a bond.(G.2) 2.What kind of n bonds are needed to delocalize the n electrons among all the main atoms in a molecule?Must all unhybridized orbitals be in the same direction?(G.7) 3.The following sentence is from the textbook Because B-carotene contains 11 conjugated double bonds,its n electrons are very extensively delocalized.We want to know whether the bonds in B-carotene are double bonds or not.If they are,why it still says n electrons are very extensively delocalized."We think double bonds'n electrons are localized.(G.2)
1. “Why Azobenzene is said to have greater delocalization of its π electrons than hydrazobenzene?” We don’t know why the π electrons of N should be delocalized? We think the bond between N-N is just a double bond rather than a delocalized π bond. (G. 2) 2. What kind of π bonds are needed to delocalize the π electrons among all the main atoms in a molecule? Must all unhybridized Questions in your team work reports : orbitals be in the same direction? (G. 7) 3. The following sentence is from the textbook :Because β-carotene contains 11 conjugated double bonds, its π electrons are very extensively delocalized. We want to know whether the bonds in β-carotene are double bonds or not. If they are, why it still says “π electrons are very extensively delocalized.” We think double bonds’ π electrons are localized. (G. 2)
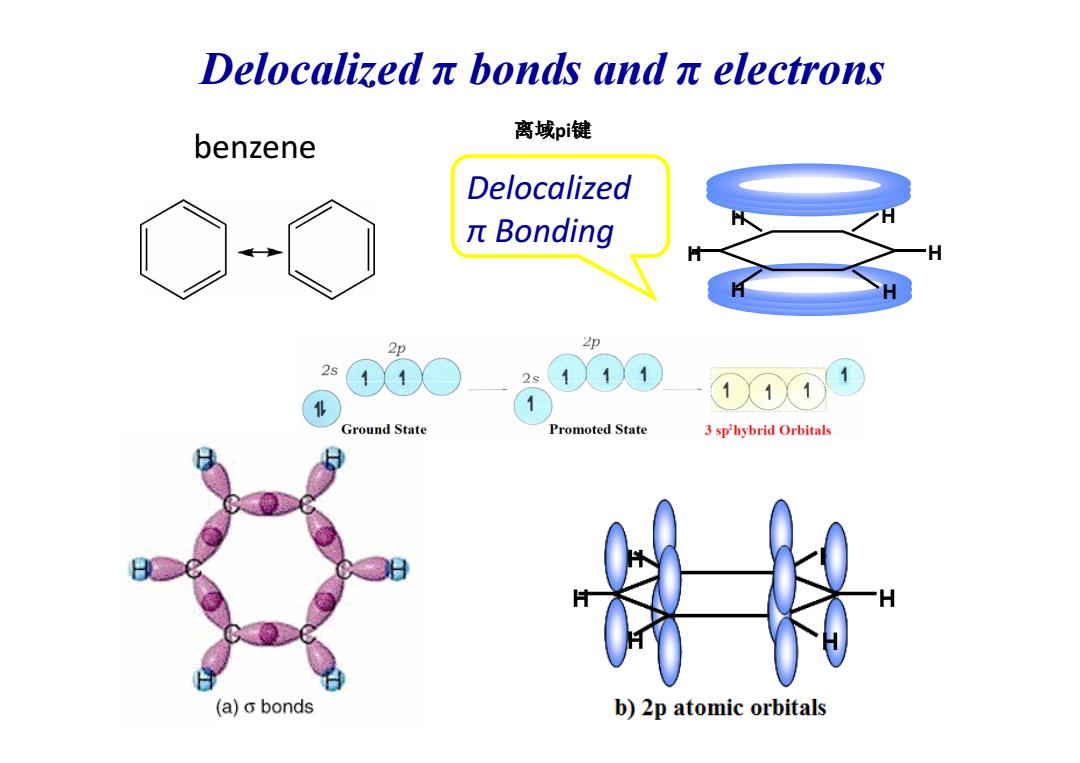
Delocalizedπbonds andπelectrons 离域pi键 benzene Delocalized πBonding 2p 2p 2s 111 Ground State Promoted State 3 sphybrid Orbitals (a)o bonds b)2p atomic orbitals
benzene H H H H H H Delocalized π Bonding 离域pi键 Delocalized π bonds and π electrons
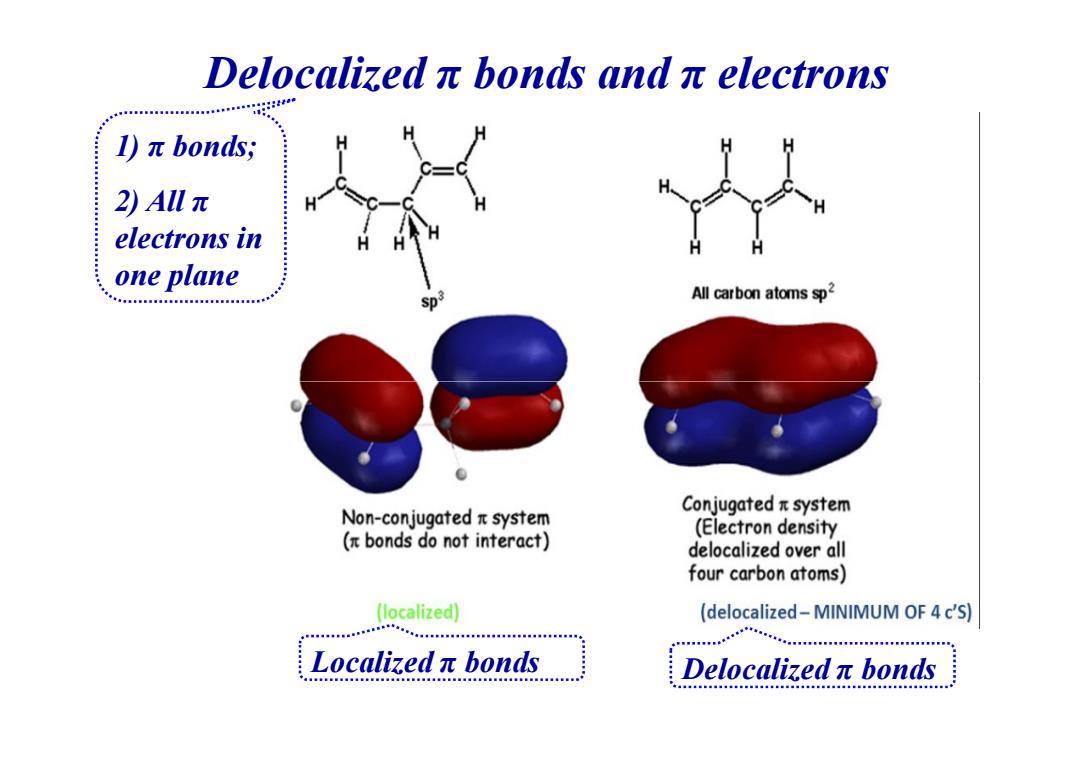
Delocalizedπbonds andπelectrons )元bonds;. 2)Allπ electrons in one plane Sp3 All carbon atoms sp2 Non-conjugated x system Conjugatedπsystem (Electron density bonds do not interact) delocalized over all four carbon atoms) (localized) (delocalized-MINIMUM OF 4 c'S) Localizedπbonds Delocalizedπbonds
Delocalized π bonds and π electrons 1) π bonds; 2) All π electrons in one plane Localized π bonds Delocalized π bonds
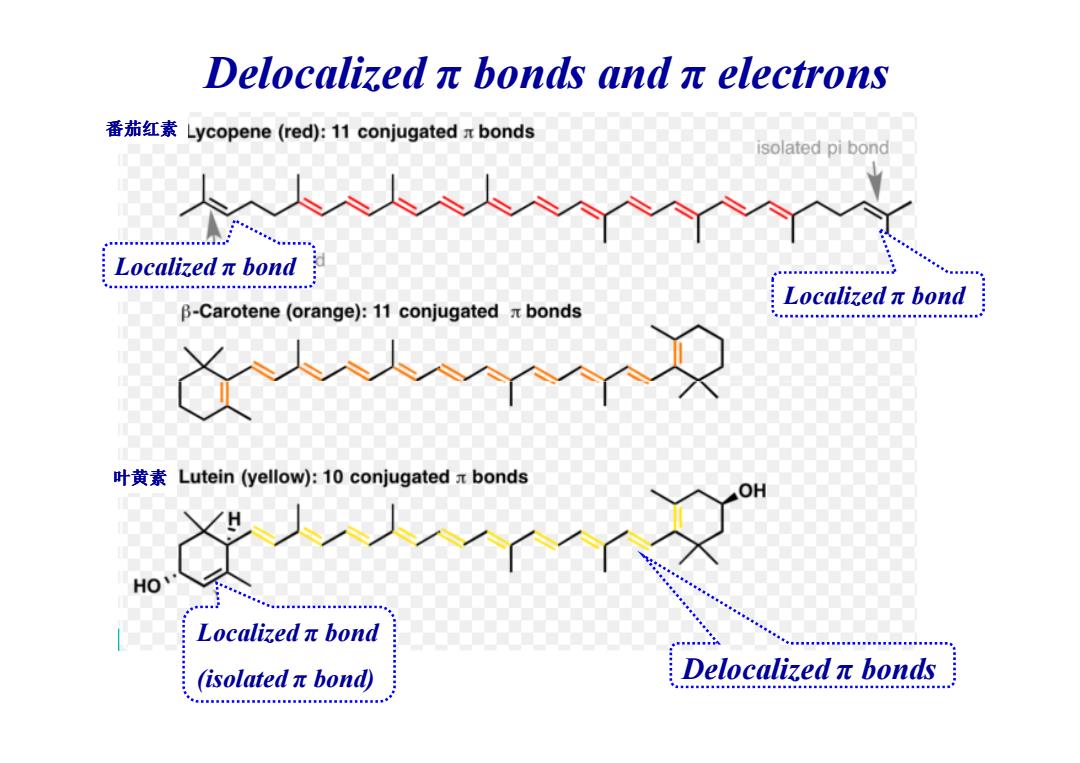
Delocalizedπbonds andπelectrons 番茄红素Lycopene(red:11 conjugated x bonds isolated pi bond Localizedπbond a年8s年0s048年8ss8年年s888esa Localizedπbond B-Carotene (orange):11 conjugated x bonds 叶黄素Lutein(yellow):10 conjugated x bonds OH Localizedπbond isolatedπbond edπbon Delocalizedπbonds
Delocalized π bonds and π electrons 番茄红素 Localized π bond Localized π bond Localized π bond (isolated π bond) Delocalized π bonds 叶黄素