Molecular Geometry and Bonding Theories 9.4 Covalent Bonding and Orbital Overlap 9.5 Hybridization Hybrid Orbitals 9.6 Multiple bonds 9.7 Molecular Orbitals Chapter 9
Molecular Geometry and Bonding Theories Chapter 9 9.4 Covalent Bonding and Orbital Overlap 9.5 Hybridization & Hybrid Orbitals 9.6 Multiple bonds 9.7 Molecular Orbitals

NaCl Others? Covalent bonds.! alibaba.com.cn
NaCl Others? Covalent Bonds!
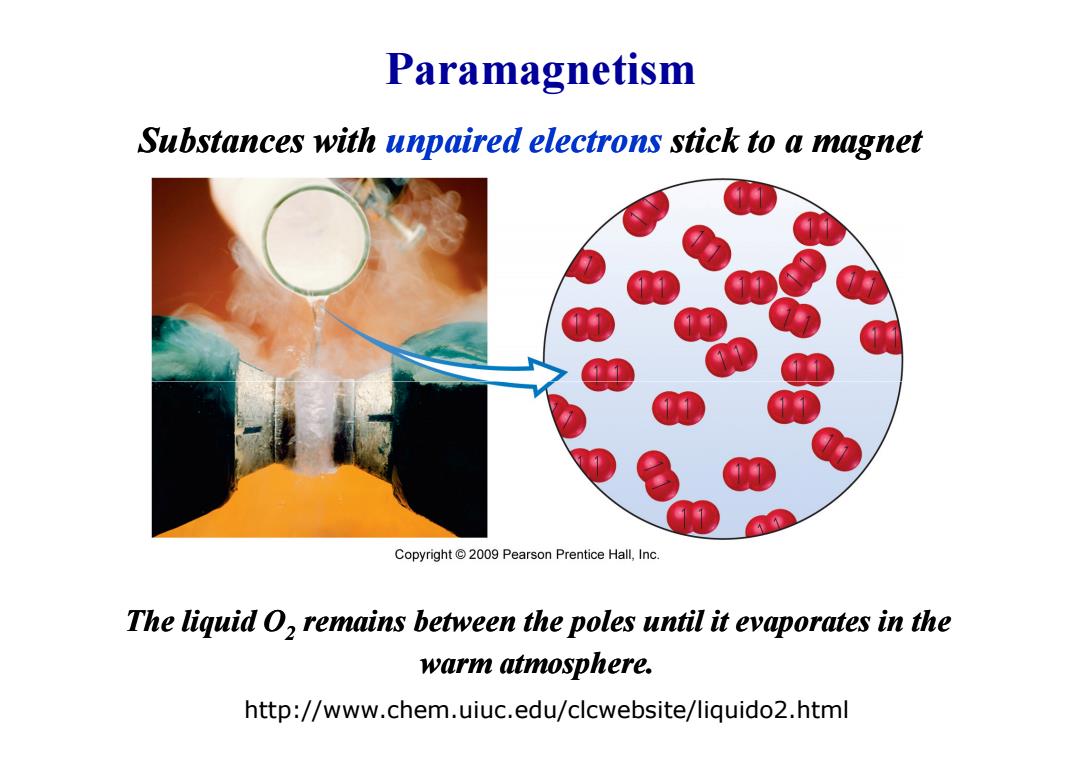
Paramagnetism Substances with unpaired electrons stick to a magnet Copyright 2009 Pearson Prentice Hall,Inc. The liquid O,remains between the poles until it evaporates in the warm atmosphere. http://www.chem.uiuc.edu/clcwebsite/liquido2.html
Paramagnetism Substances with unpaired electrons stick to a magnet http://www.chem.uiuc.edu/clcwebsite/liquido2.html The liquid O2 remains between the poles until it evaporates in the warm atmosphere
Molecular Orbital (MO)Theory What's a MO Atomic Orbitals (AO) Wave functions ( Molecular Orbitals(MO) Different‘shapes': -MO:o (sigma)and (pi) -AO:s,p,d,f etc
Molecular Orbital (MO) Theory Atomic Orbitals ( ) Wave functions ( ) Molecular Orbitals ( ) A MO O ψ Different ‘shapes’: – MO: σ (sigma) and π (pi) – AO: s, p, d, f, etc
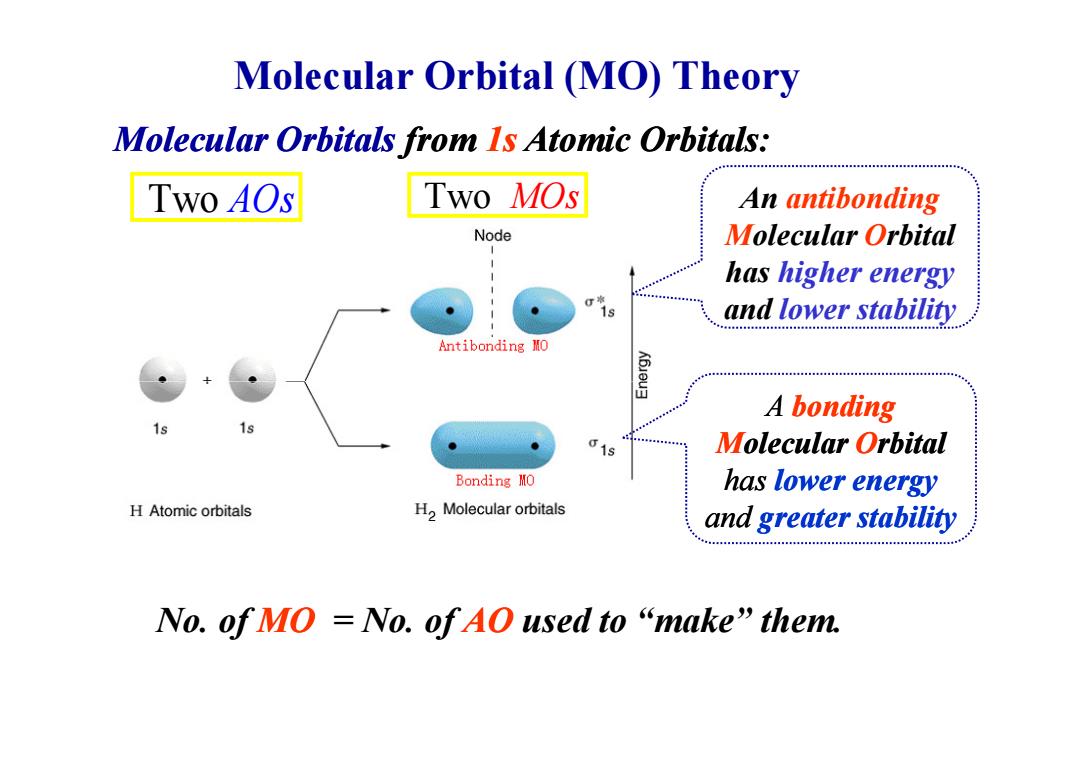
Molecular Orbital (MO)Theory Molecular Orbitals from Is Atomic Orbitals: Two A0s Two MOs An antibonding Node Molecular Orbital has higher energy s and lower stability Antibonding MO A bonding 61 Molecular Orbital Bonding MO has lower energy H Atomic orbitals H2 Molecular orbitals and greater stability No.ofMO=No.ofAO used to“make”them
Molecular Orbital (MO) Theory Two AOs Two MOs An antibonding Molecular Orbital has higher energy and lower stability Molecular Orbitals from 1s Atomic Orbitals: No. of MO = No. of AO used to “make” them. A bonding Molecular Orbital has lower energy and greater stability