
Chapter 8 Basic Concepts of Chemical Bonding 8.I Chemical Bonds 8.2 Ionic bond, 8.3 Covalent Bonding 8.4 Bond polarity electronegativ ty 8.5 Lewis Structure
Chapter 8 Basic Concepts of Chemical Bonding 8.1 Chemical Bonds 8.2 Ionic bond, 8.3 Covalent Bonding 8.4 Bond polarity & 8.4 Bond polarity & electronegativity electronegativity 8.5 Lewis Structure

Hard-soft:hardness; Electron conductor-semiconductor-Physical insulator:conductivity; properties Solid liquidgas:phase or states; Chemical Poisonous-safe:chemical reactivity. alibaba.com.cn crscientito.com
Hard—soft: hardness; Electron conductor—semiconductor— insulator: conductivity; Physical properties Chemical insulator: conductivity; Solid—liquid—gas: phase or states; Poisonous—safe: chemical reactivity. properties Chemical
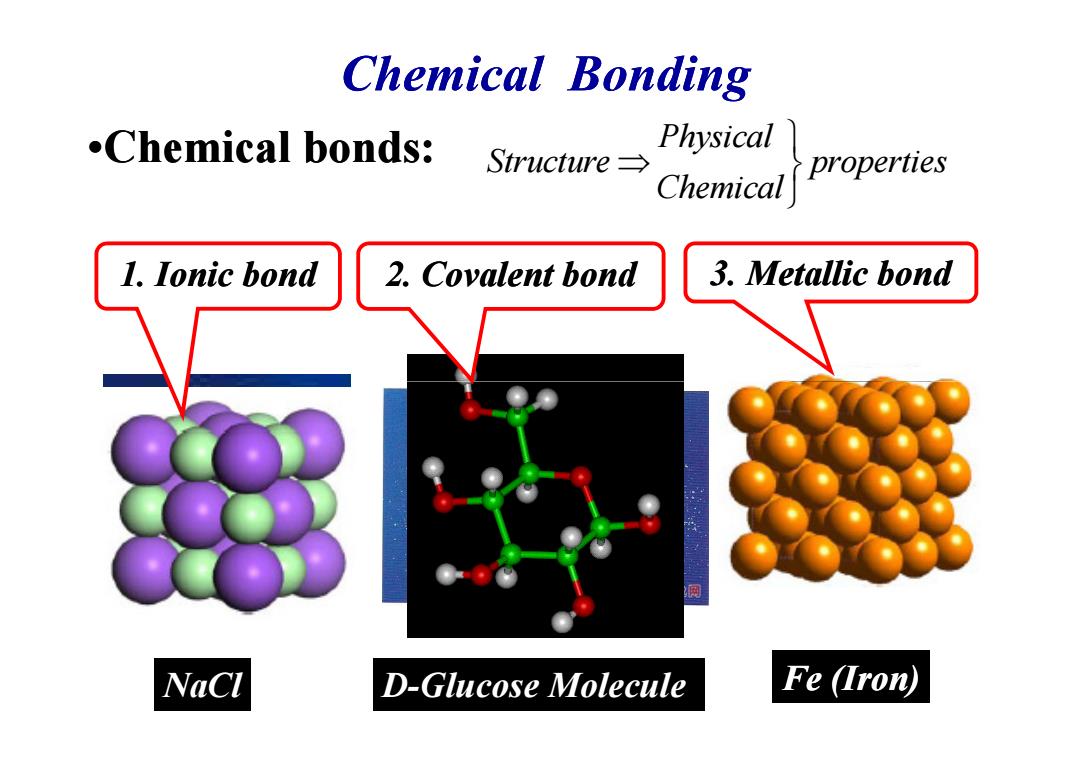
Chemical bonding .Chemical bonds: Physical Structure→ Chemical properties 1.Ionic bond 2.Covalent bond 3.Metallic bond NaCl D-Glucose Molecule Fe (Iron)
Chemical Bonding •Chemical bonds: 1. Ionic bond 2. Covalent bond 3. Metallic bond Physical Structure properties Chemical ⇒ NaCl D-Glucose Molecule Fe (Iron)
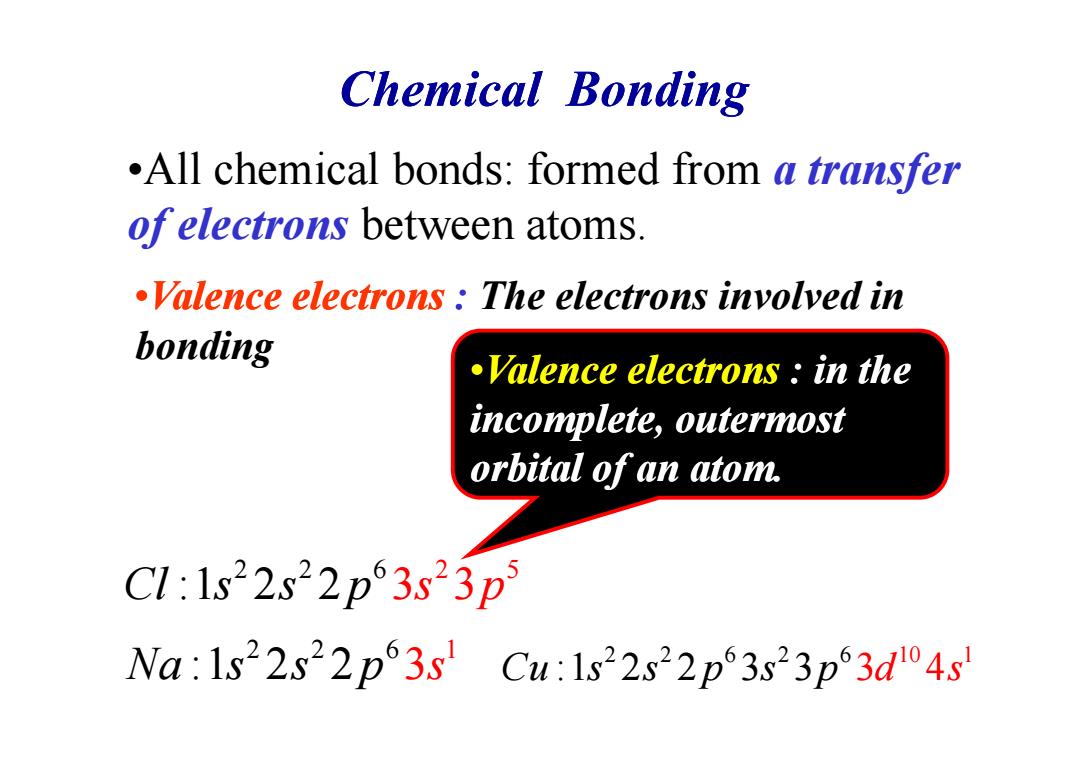
Chemical bonding .All chemical bonds:formed from a transfer ofelectrons between atoms. Valence electrons The electrons involyed in bonding Valence electrons in the incomplete,outermost orbital of an atom. C1:1s22s22p63s23p Na:1s22s22p3s Cu:1s22s22p63s23p3d4s
Chemical Bonding •All chemical bonds: formed from a transfer of electrons between atoms. •Valence electrons : The electrons involved in bonding •Valence electrons : in the 2 2 6 1 Na s s :1 2 2 p 3 s 2 6 2 2 5 C s s p l :1 2 2 3 3 s p 2 2 6 2 6 10 1 C s s p s p u :1 2 2 3 3 3 4 d s •Valence electrons : in the incomplete, outermost orbital of an atom
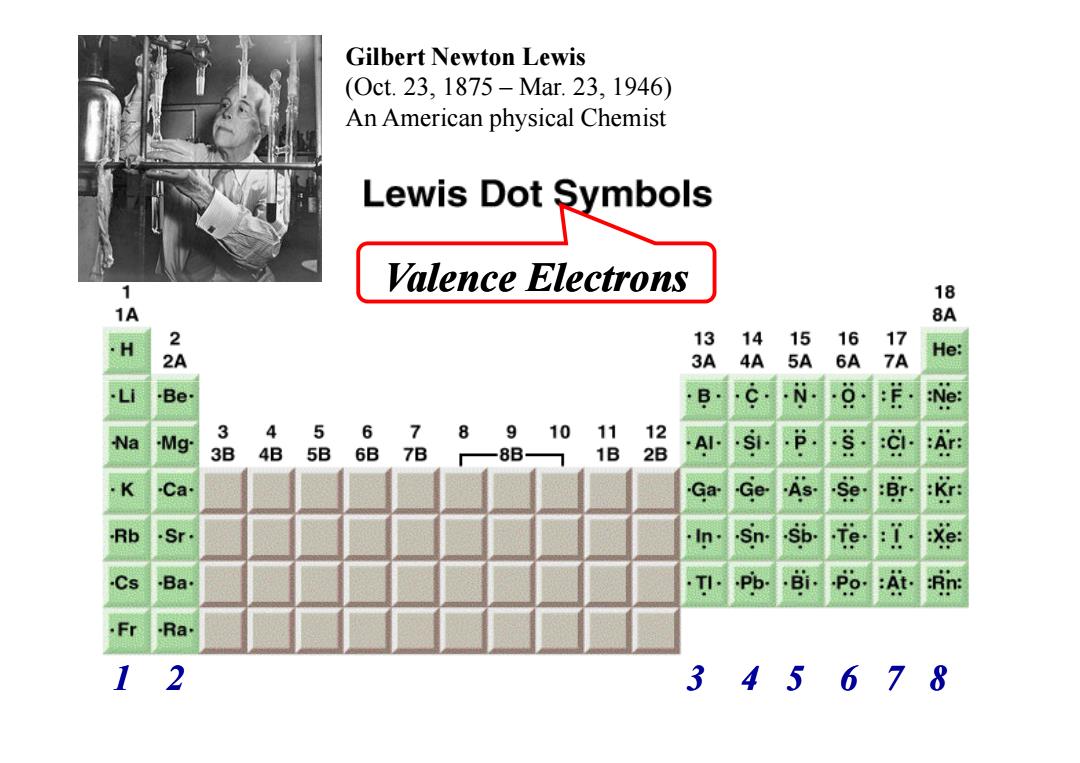
Gilbert Newton Lewis (0ct.23,1875-Mar.23,1946) An American physical Chemist Lewis Dot Symbols Valence electrons 18 1A 8A 2 13 14 15 16 17 3A 4A 5A 6A 7A He: Be B -N- 0 :Ne: 4 5 6 7 8 Na -Mg 3 91011 12 .P 3B 4B 5B 6B 7B —8B 1B 2B -AI-.SI- A K Ca Ga Ge.As.se.:Br. K Rb Sr .In..sn.sb.fe.:1. :Xe: Cs .Ba. TI..Pb..Bi.Po.At. :Rn: Fr Ra 2 345678
Valence Electrons Gilbert Newton Lewis (Oct. 23, 1875 – Mar. 23, 1946) An American physical Chemist 1 2 3 4 5 6 7 8