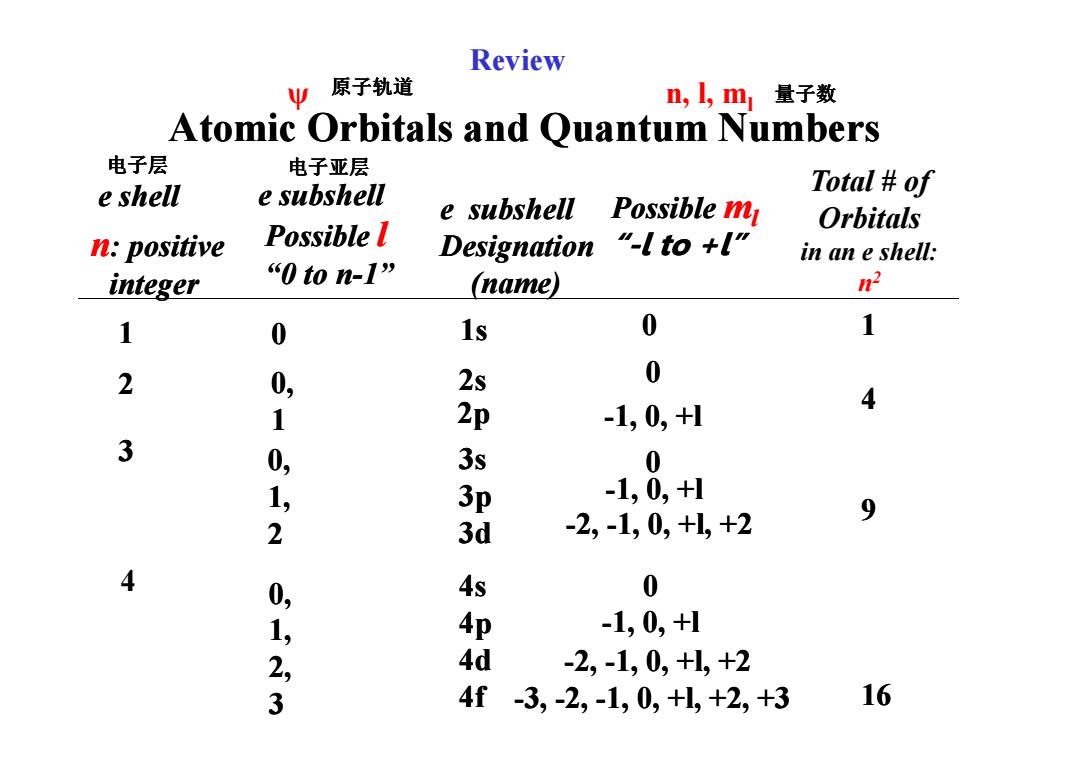
Review Ψ 原子轨道 n,,m量子数 Atomic Orbitals and Quantum Numbers 电子层 电子亚层 e shell e subshell Total of e subshell Possible m Orbitals n:positive Possible l Designation "-l to +l" in an e shell: integer “0ton-l” (name) n2 1 0 1s 0 1 2 0, 2s 0 2p 4 1 -1,0,+1 3 0, 3s 0 1, 3p -1,0,+1 -2,-1,0,+l,+2 9 2 3d 4 0, 4s 0 1, 4p -1,0,+1 2, 4d -2,-1,0,+1,+2 3 4f -3,-2,-1,0,+1,+2,+3 16
Atomic Orbitals and Quantum Numbers e shell 12 Possible l “0 to n “0 to n-1” 0 0, n: positive integer 1s 2s Possible ml “-l to +l” l to +l” 00 Total # of Orbitals in an e shell: n 2 14 ψ n, l, ml Review e subshell subshell e subshell subshell Designation (name) 原子轨道 量子数 电子层 电子亚层 23 4 0, 1 0, 1, 2 0, 1, 2, 3 2s 2p 3s 3p 3d 4s 4p 4d 4f 0 -1, 0, +l 1, 0, +l 0 -1, 0, +l 1, 0, +l -2, -1, 0, +l, +2 1, 0, +l, +2 0 -1, 0, +l 1, 0, +l -2, -1, 0, +l, +2 1, 0, +l, +2 -3, -2, -1, 0, +l, +2, +3 1, 0, +l, +2, +3 49 16
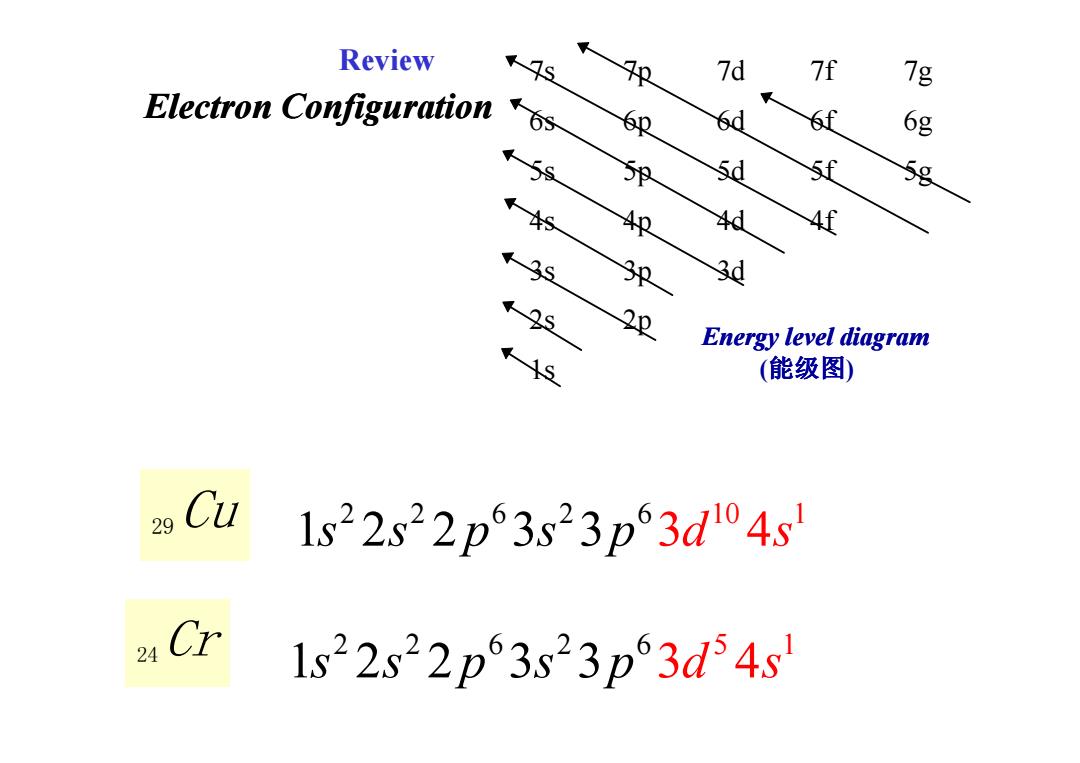
Review 9s 7d 7f 7g Electron Configuration 6p 6g 59 5d 58 4牧 4d 4f 3d Energy level diagram (能级图) 29 Cu 1s22s22p63,s23p63dl04s Cr 1s22s22p63s23p3d4s
Electron Configuration 7s 7p 7d 7f 7g 6s 6p 6d 6f 6g 5s 5p 5d 5f 5g 4s 4p 4d 4f 3s 3p 3d 2s 2p 1s Energy level diagram (能级图 ) Review 29Cu 24 Cr 2 2 6 2 6 10 1 1s s p s p 2 2 3 3 3d s 4 2 2 6 2 6 5 1 1 2 2 3 3 s s p s p 3 4 d s 1s ( )
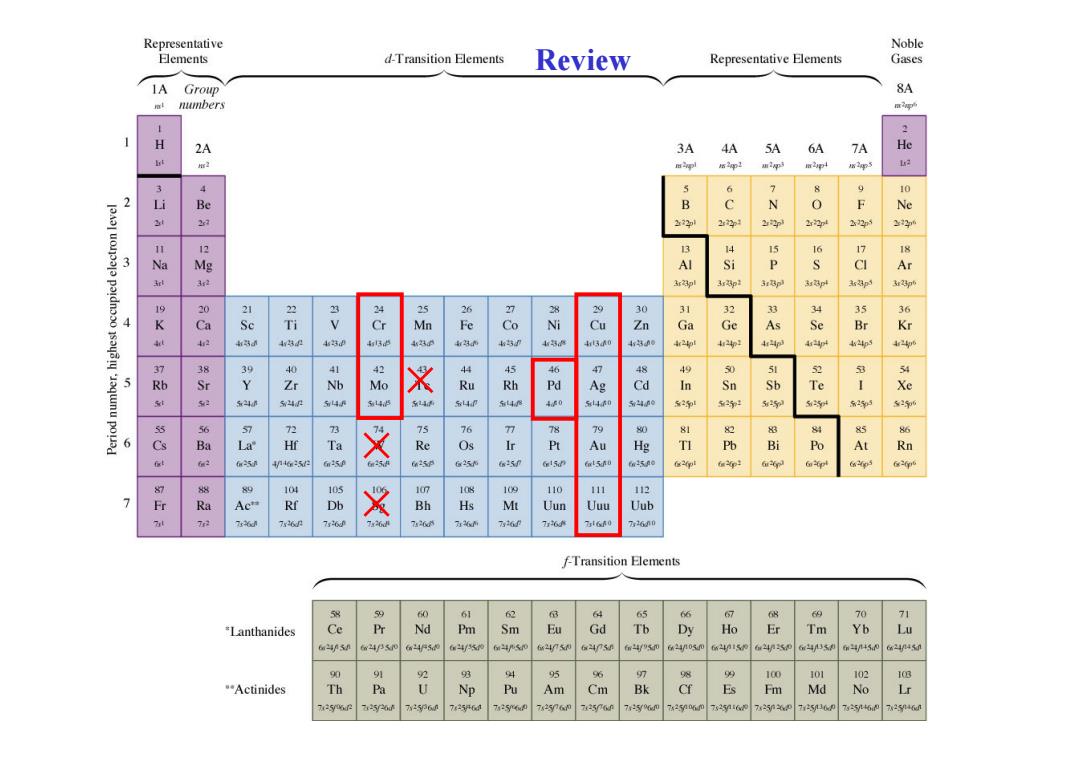
Representative Noble Elements d-Transition曰ements Review Representative Elements Gases IA Group 8A numbers 1 2 H 2A 3A 4A 5A 6A 7A He s2gp1 通2p m2p 3 4 6 2 Be N 0 F Ne 2学1 22p 22p 22 12 13 3 Si P A 3rBpl 23 26 7 0 A 9K8 R 83 Ti & Ni Cu Zn Ga Ge As 4特8 450 44p 新 5 8分 Y m 品 4 t48 314 6 84 A知 品 B Po m 625 625 105 110 111 112 7 行 A Bh Uun Uuu Uub 7 76 7r60 2 7426 76 76 t60 26n0 F-Transition Elements 58 1 6 65 69 70 71 *Lanthanides r Nd Pm Sm Eu Gd Dy Ho Er Tm Yb Lu 6m5n 2450 24n050 62415 6J5 6m/45M 6a2445n 90 2 男 94 95 96 98 100 101 1(B Actinides Th Pa Am Cm Bk Es Fm Md No Lr 5 7256n 7e256d 7a250 72n 75y0 7250 7206 7252 75y60 7a246n
× Review × ×
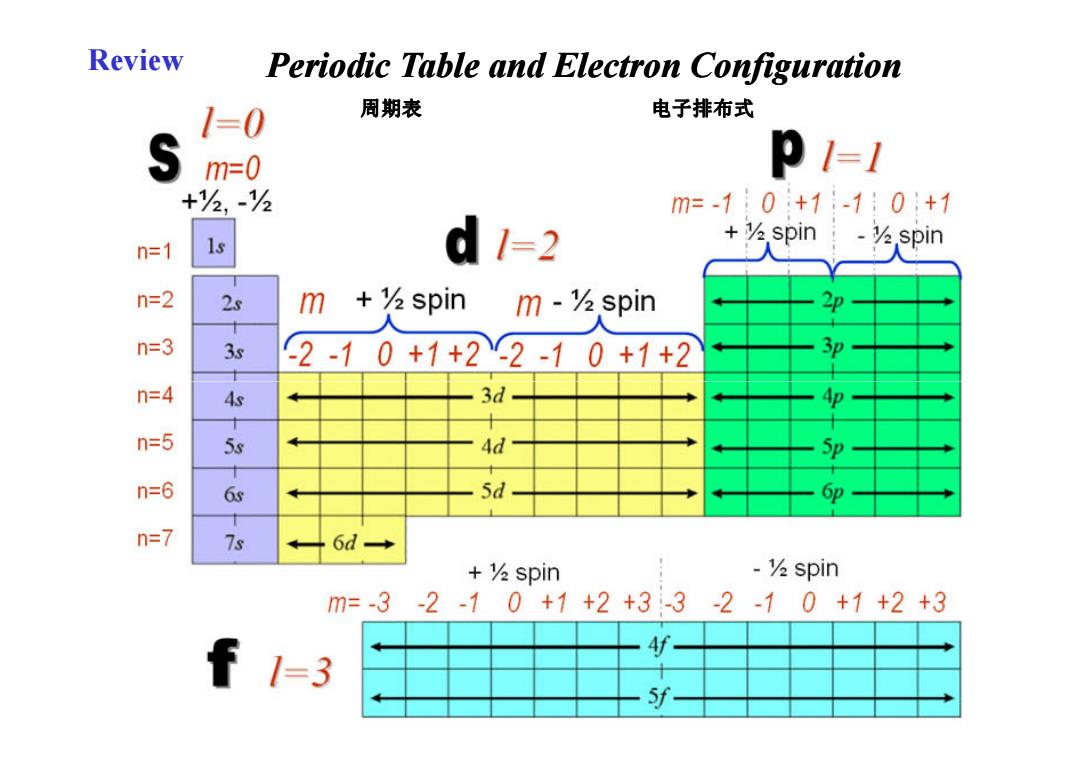
Review Periodic Table and Electron Configuration 1=0 周期表 电子排布式 m=0 p1=1 +么,-h m=-10+1-10+1 +spin V.spin n=1 1s d1=2 n=2 25 m +spin m-么spin n=3 3s 2-10+1+22-10+1+2 3P n=4 As 3d 十 n=5 5s Ad 5p n=6 Gs 5d 6p 十 n=7 7s 6d→ +%spin -V spin m=-3-2-10+1+2+3-3-2-10+1+2+3 f 1=3 斯 十 5f-
Review 周期表 Periodic Table and Electron Configuration 电子排布式

Review The size ofAtoms,ions and Molecules Trends in atomic radii. A Na (10-10m) 3 2 Nb Mo Co Ru .15 1A2A Rh G1.3 31 5 h.48 9 Transition metals Increasing radius 3AAAGA6A7A8A Increasing radius Li+ Li Copyright 2009 Pearson Prentice Hall,Inc. 0.90 1.34
Trends in atomic radii. The size of Atoms, ions and Molecules Review Å (10-10 m)