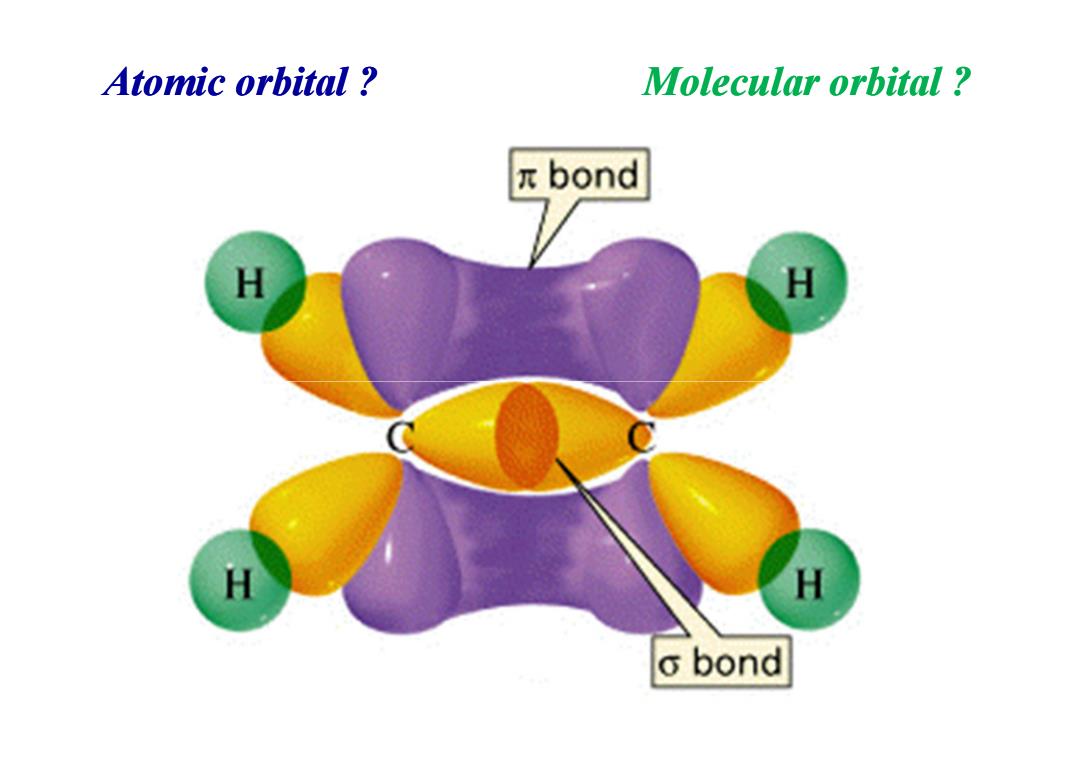
Atomic orbital Molecular orbital πbond H H H H o bond
Atomic orbital ? Molecular orbital ?
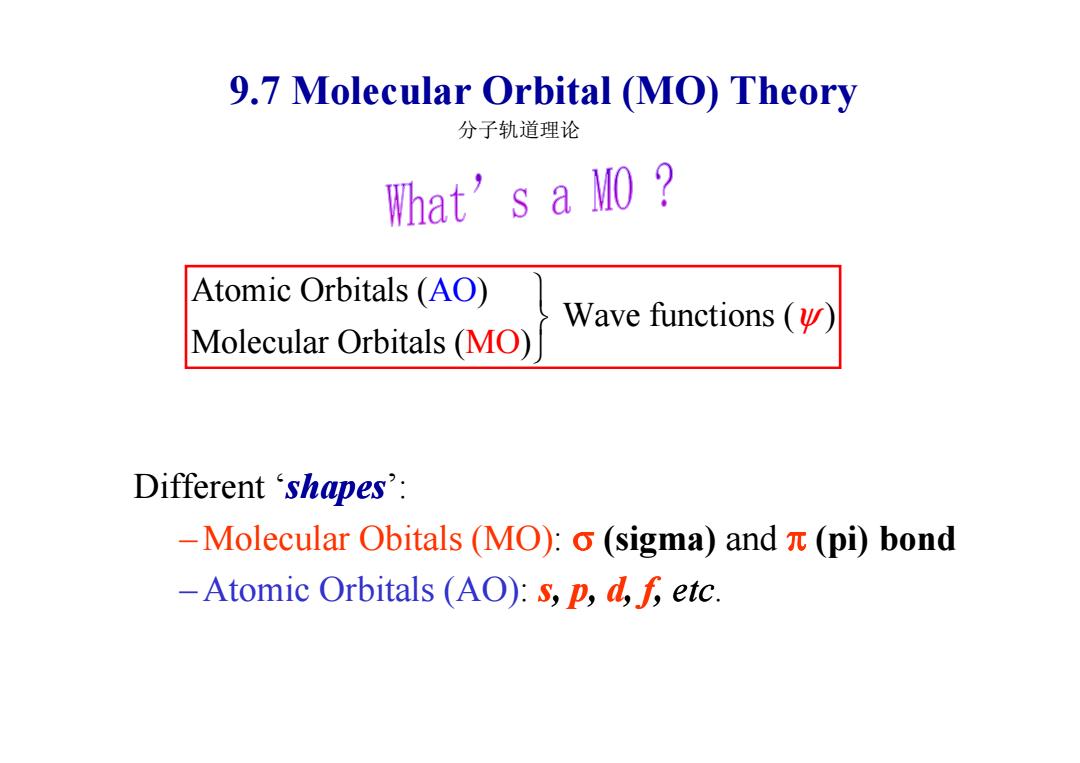
9.7 Molecular Orbital (MO)Theory 分子轨道理论 What's a MO Atomic Orbitals (AO) Wave functions ( Molecular Orbitals (MO) Different‘shapes': -Molecular Obitals (MO):o(sigma)and (pi)bond -Atomic Orbitals (AO):s,p,d,f,etc
9.7 Molecular Orbital (MO) Theory Atomic Orbitals ( ) Wave functions ( ) Molecular Orbitals ( ) A MO O ψ 分子轨道理论 Different ‘shapes’: – Molecular Obitals (MO): σ (sigma) and π (pi) bond – Atomic Orbitals (AO): s, p, d, f, etc

9.7 Molecular Orbital (MO)Theory 分子轨道理论 Molecular Orbitals from Is Atomic Orbitals: Two A0s Two MOs An antibonding Node Molecular Orbital has higher energy s and lower stability Antibonding MO 反键轨道 A bonding 01 Molecular Orbital Bonding MO成键轨道 has lower energy H Atomic orbitals H2 Molecular orbitals and greater stability No.ofMO=No.ofAO used to“make”them
9.7 Molecular Orbital (MO) Theory Two AOs Two MOs An antibonding Molecular Orbital has higher energy and lower stability Molecular Orbitals from 1s Atomic Orbitals: 分子轨道理论 反键轨道 No. of MO = No. of AO used to “make” them. A bonding Molecular Orbital has lower energy and greater stability 反键轨道 成键轨道

Molecular Orbitals in H2 Why H2 is more stable than individual H atoms? Node Antibonding MO K6ieu3 Energy-level diagram(能级图) 015 1s Bonding MO H Atomic orbitals H2 Molecular orbitals 1s 1s H atom H atom 2 bonding electrons keep the two atoms H:molecule together. olecular orbital diagram(分子轨道能力图)
Molecular Orbitals in H2 Why H2 is more stable than individual H atoms? Energy-level diagram level diagram (能级图) 2 bonding electrons keep the two atoms together. Molecular orbital diagram (分子轨道能力图)

9.7 Molecular Orbital (MO)Theory Electron configuration of quantized MO: Lowest energy rule ---Lowest energy level fills first Pauli's Exclusion Principle ---2e maximum per orbital Hund's rule --For degenerate orbitals 1 electron in each orbital before double-up!
9.7 Molecular Orbital (MO) Theory Lowest energy rule --- Lowest energy level fills Lowest energy level fills first Electron configuration of quantized MO : Pauli’s Exclusion Principle --- 2e maximum per 2e maximum per orbital Hund’s rule --- For degenerate orbitals : 1 electron in each orbital before double electron in each orbital before double-up!