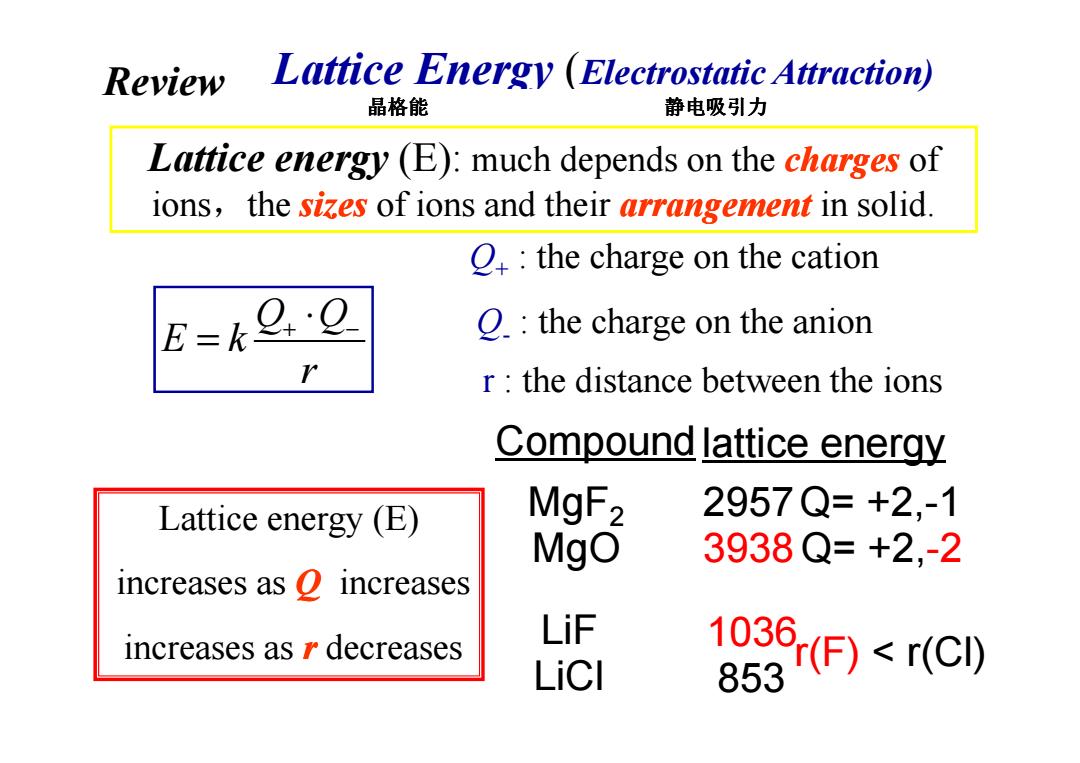
Review Lattice Energy (Electrostatic Attraction) 晶格能 静电吸引力 Lattice energy (E):much depends on the charges of ions,the sizes of ions and their arrangement in solid. O the charge on the cation E=k O.the charge on the anion r the distance between the ions Compound lattice energy Lattice energy (E) MgF2 2957Q=+2,-1 MgO 3938Q=+2,-2 increases as o increases LiF increases as r decreases LiCI (F)r(C) 1036
Lattice Energy (Electrostatic Attraction) Q+ : the charge on the cation Q- : the charge on the anion r : the distance between the ions Lattice energy (E): much depends on the charges of ions,the sizes of ions and their arrangement in solid. Q Q E k r + − ⋅ = 晶格能 静电吸引力 Review Lattice energy (E) increases as Q increases increases as r decreases lattice energy MgF2 MgO LiF LiCl 2957 3938 1036 853 Q= +2,-1 Q= +2,-2 r(F) < r(Cl) r : the distance between the ions Compound r
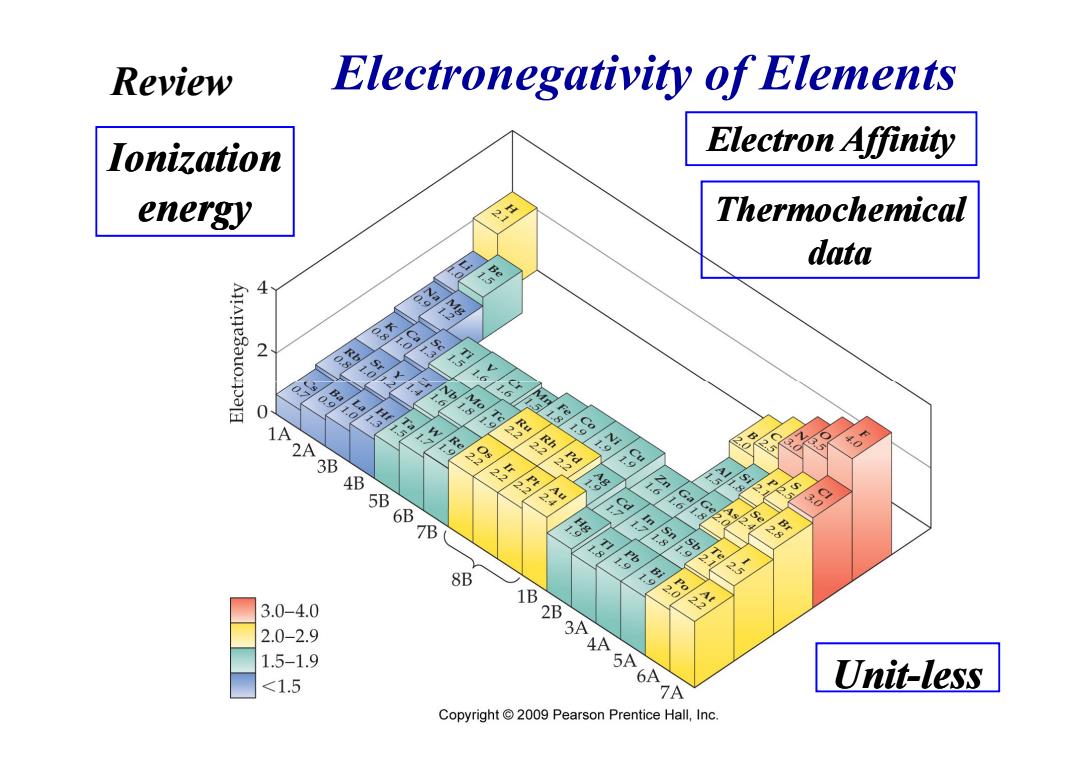
Review Electronegativity of Elements Ionization Electron Affinity energy Thermochemical data 系 39 0 88 的容 8 3B 68 1.5 8 59 e 9 6带 0 9 的平 8 8B 一 3.0-4.0 已之 2.0-2.9 1.5-1.9 5 <1.5 61P Unit-less Copyright 2009 Pearson Prentice Hall,Inc
Figure 08.06 Electronegativity of Elements Electron Affinity Ionization energy Thermochemical data Review Unit-less
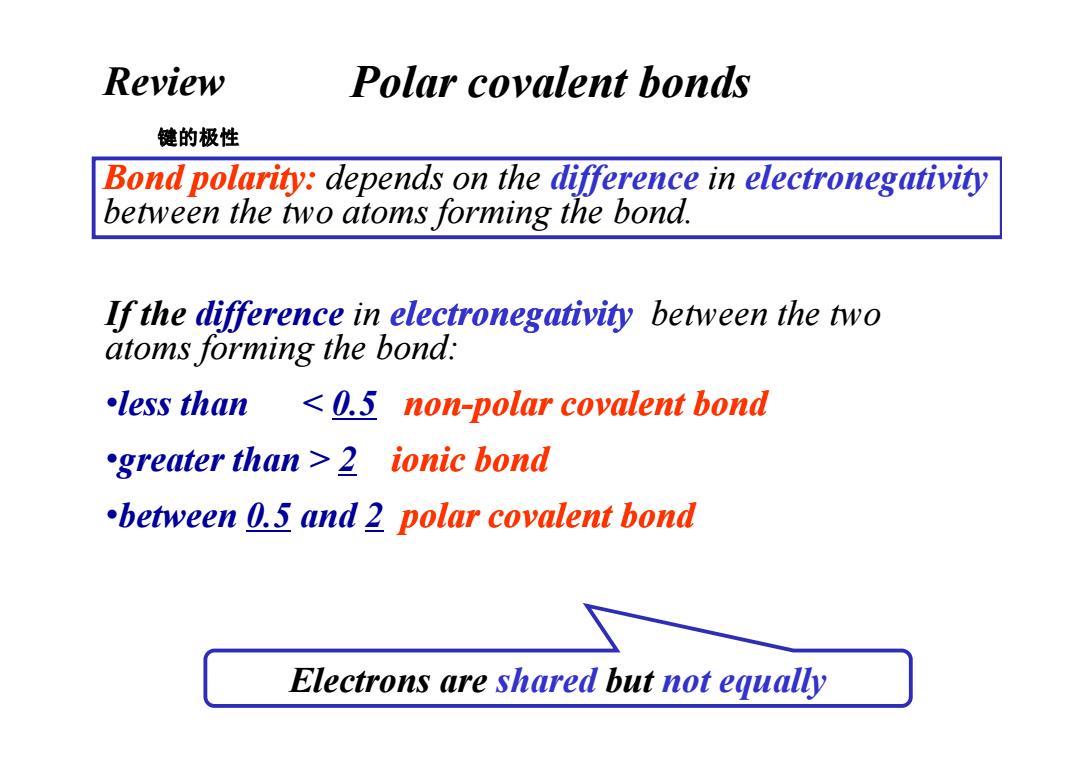
Review Polar covalent bonds 键的极性 Bond polarity:depends on the difference in electronegativity between the two atoms forming the bond. If the difference in electronegativity between the two atoms forming the bond: less than 0.5 non-polar covalent bond greater than 2 ionic bond between 0.5 and 2 polar covalent bond Electrons are shared but not equally
Polar covalent bonds Bond polarity: depends on the difference in electronegativity between the two atoms forming the bond. If the difference in electronegativity between the two atoms forming the bond: 键的极性 Review •less than < 0.5 non-polar covalent polar covalent bond •greater than > 2 ionic bond •between 0.5 and 2 polar covalent bond Electrons are shared but not equally
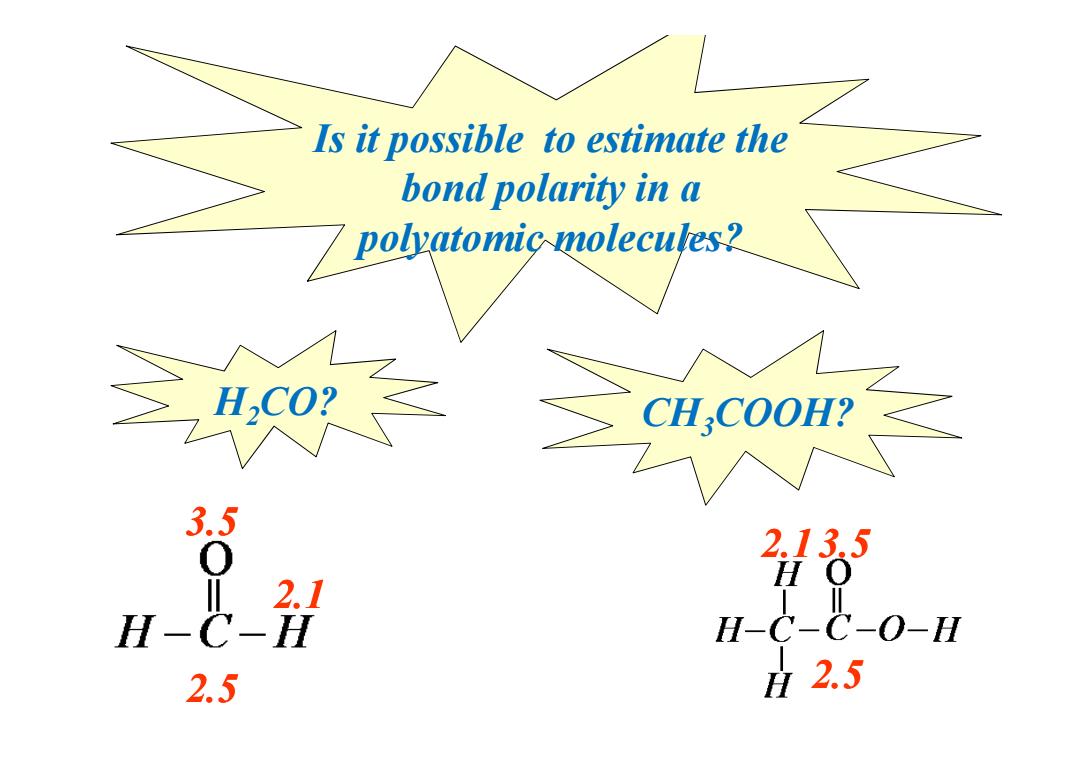
Is it possible to estimate the bond polarity in a 7 polyatomic molecules? CHCOOH? 3.5 2.135 心H H- H-C-C-O-H 2.5 2.5
Is it possible to estimate the bond polarity in a polyatomic molecules? H2CO? CH3COOH? 2.1 3.5 2.5 3.5 2.5 2.1

Solid at R.T; Lattice Hard and brittle; Energy: Conductivity? +788kJ/mol NaCl Strength of ionic bonds E=k alibaba.com.cn
Solid at R. T.; Hard and brittle; Conductivity? Lattice Energy: +788kJ/mol NaCl Q Q E k r + − ⋅ = Strength of ionic bonds E k r