
The Wave Behavior of Matter Matter wave---de Broglie's wave h mv To study small objects: a)X-ray diffraction b)Electron diffraction The discovery ofelectron diffraction by crystals in 1927 Diffraction pattern of by Dayvisson and Germer electron beams
The Wave Behavior of Matter v h m λ = Matter wave Matter wave---de Broglie’s wave de Broglie’s wave To study small objects: a) X-ray diffraction b) Electron diffraction Diffraction pattern of electron beams The discovery of electron diffraction by crystals in 1927 by Dayvisson and Germer
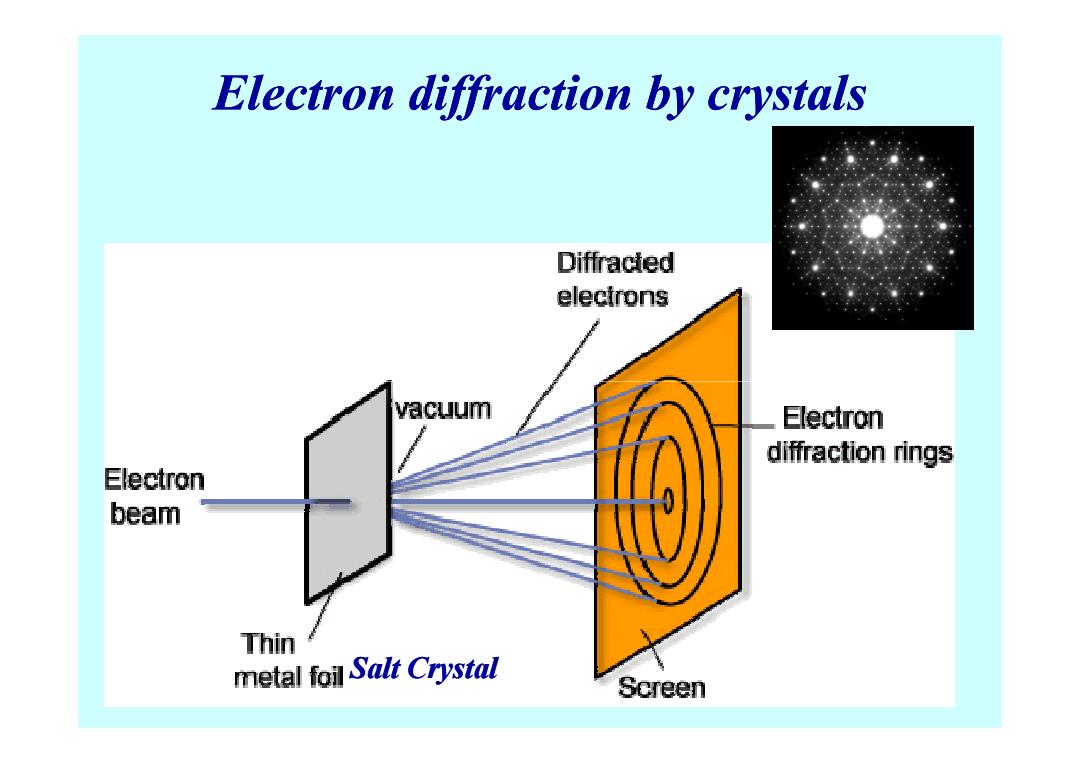
Electron diffraction by crystals Diffracled electrons vacuum Electron diffraction rings Electron beam Thin metal foil Salt Crystal Screen
Electron diffraction by crystals Salt Crystal
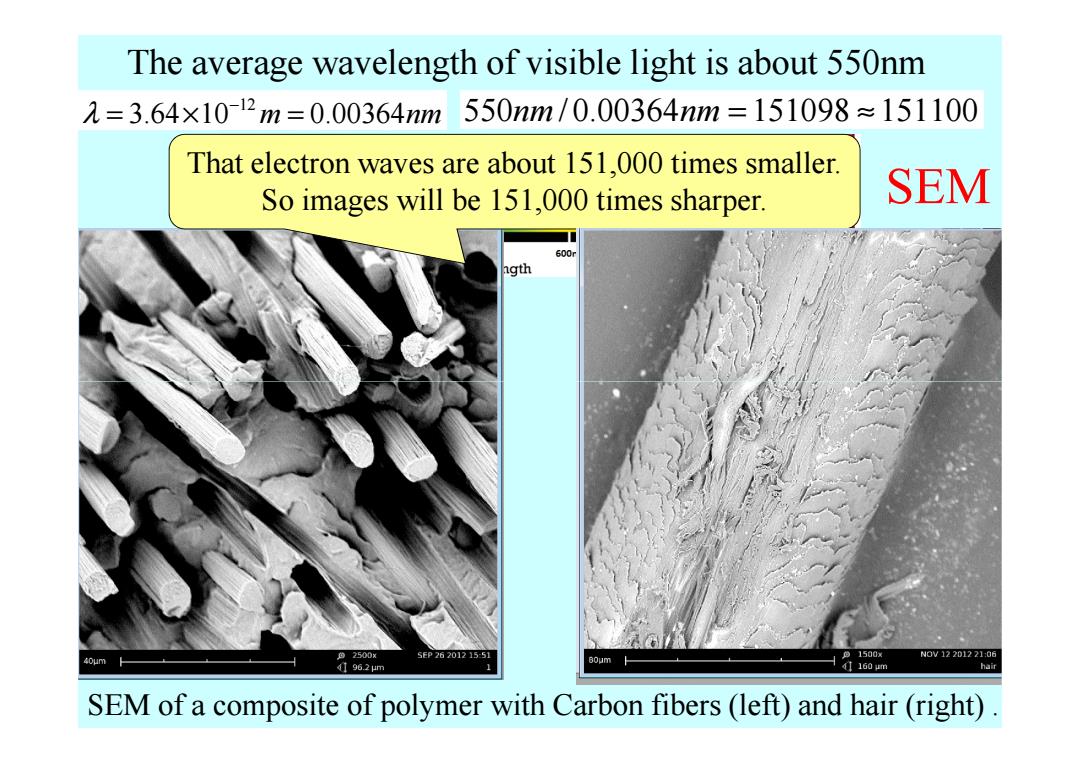
The average wavelength of visible light is about 550nm 元=3.64×1012m=0.00364m550nm/0.00364nm=151098≈151100 That electron waves are about 151,000 times smaller. So images will be 151,000 times sharper. SEM 600r ngth 色2500× SEP26201215-51 。1500x N0V12201221:06 40um 196.2μm B0um 1 41160um hair SEM of a composite of polymer with Carbon fibers(left)and hair(right)
The average wavelength of visible light is about 550nm 550 / 0.00364 151098 151100 nm nm = ≈ That electron waves are about 151,000 times smaller. So images will be 151,000 times sharper. 12 λ 3.64 10 0.00364 m nm − = × = SEM SEM of a composite of polymer with Carbon fibers (left) and hair (right)

Louis de Broglie Matter Wave h mv The 1929 Nobel Prize in physics "For his discovery of wave nature of electrons
Louis de Broglie Matter Wave h λ = The 1929 Nobel Prize in physics “For his discovery of wave nature of electrons” m v λ =