Chapter 6 Electronic Structure of Atoms 6.4 The Wave Behavior of Matter 6.5 Quantum Mechanical Atomic Orbitals 6.6 Representations of Orbitals 6.7 Many electron atoms 6.8 Electron Configurations 6.9 Electron Configurations Periodic Table Chapter 6
Chapter 6 Electronic Structure of Atoms 6.4 The Wave Behavior of Matter Chapter 6 6.5 Quantum Mechanical & Atomic Orbitals 6.6 Representations of Orbitals 6.7 Many electron atoms 6.8 Electron Configurations 6.9 Electron Configurations & Periodic Table
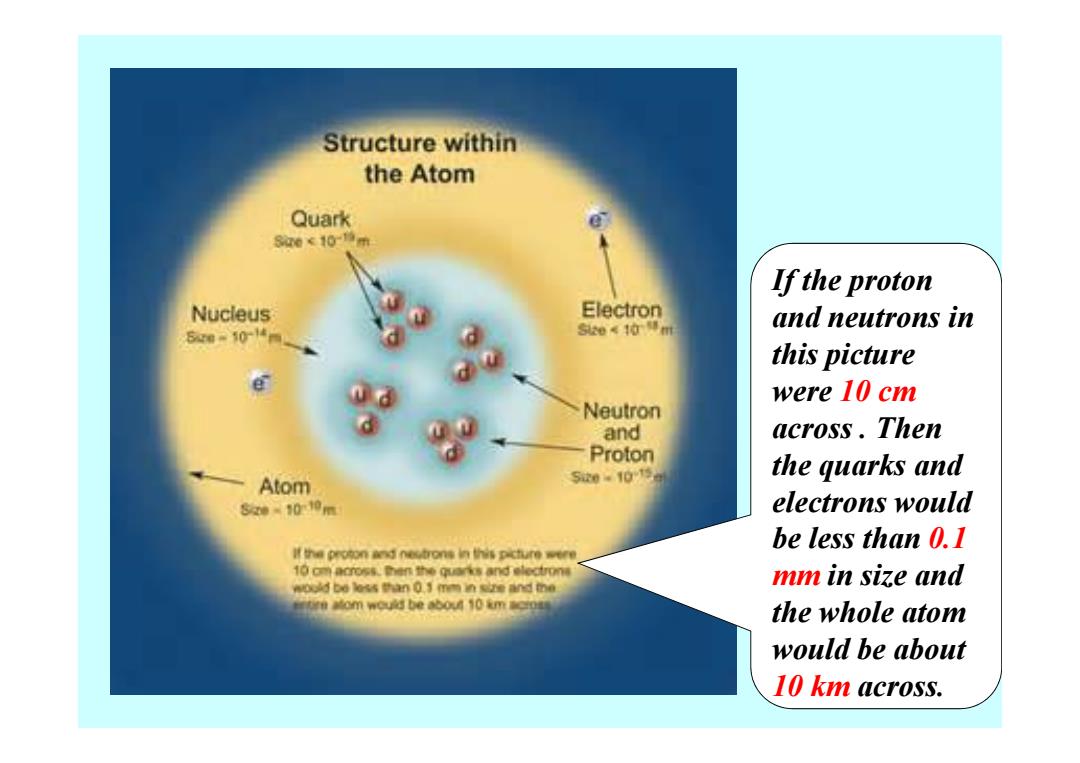
Structure within the Atom Quark 8a0<10增m If the proton Nucleus Electron and neutrons in 6m50-14% 2e《40得mt this picture were 10 cm Neutron and across.Then Proton 520.1013a the quarks and Atom 5w-100m electrons would be less than 0.I 算e proton and0ebgn裤的is picture were 10 cm across.men the quarks and electrone woold bo les5an01m两n size and me mm in size and y#om wou创be心0410mcD the whole atom would be about 10 km across
If the proton and neutrons in this picture were 10 cm across . Then the quarks and electrons would be less than 0.1 mm in size and the whole atom would be about 10 km across
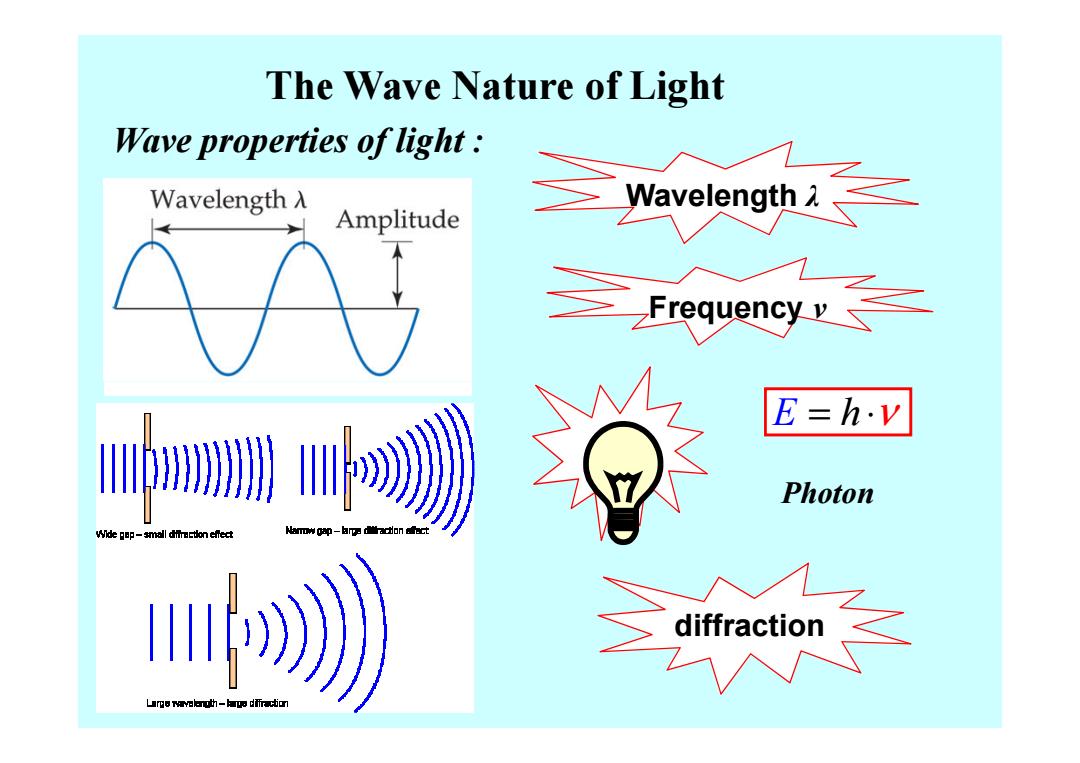
The Wave Nature of Light Wave properties of light Wavelength A Amplitude Wavelongh L E=h.v Photon Wide gop-small efec u》
The Wave Nature of Light Wave properties of light : Wavelength λ Frequency ν diffraction E = ⋅ h ν Photon
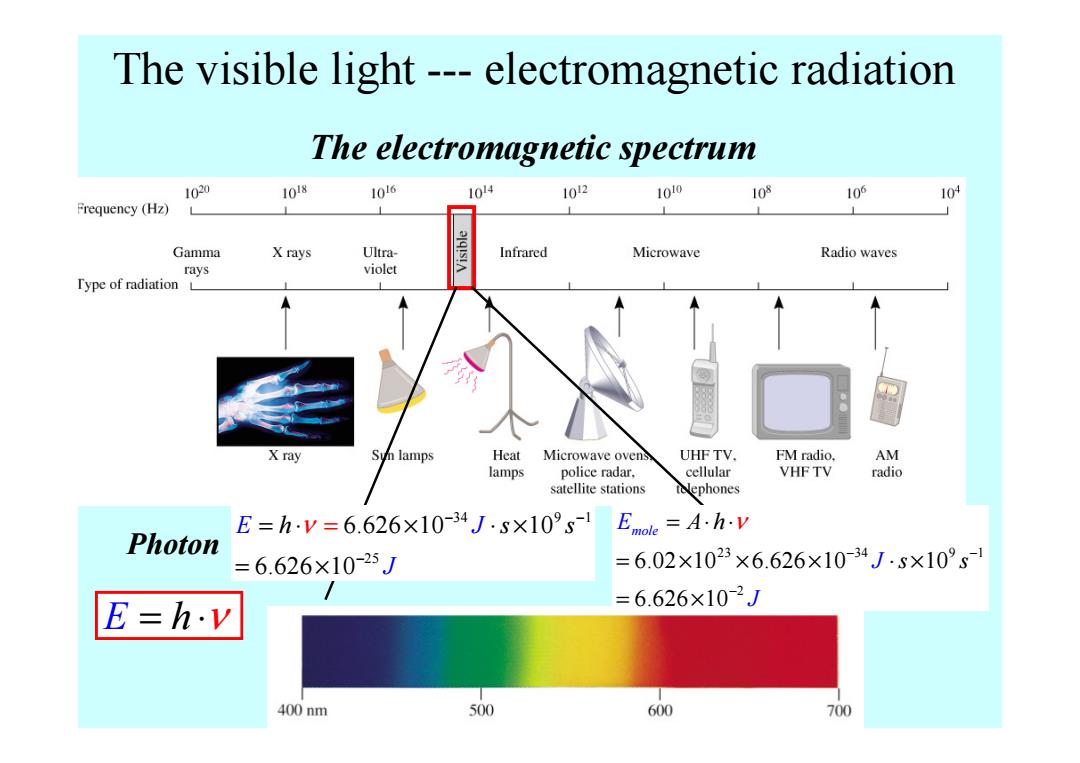
The visible light---electromagnetic radiation The electromagnetic spectrum 1020 1018 1016 1014 1012 1010 103 106 104 Frequency (Hz) Gamma X rays Ultra- alq!s!A Infrared Microwave Radio waves rays violet Type of radiation L X ray Sun lamps Heat Microwave ovens UHF TV. FM radio, AM lamps police radar. cellular VHF TV radio satellite stations tolephones E=hy=6.626×10-34J.s×109s1 Emole =A.h.v Photon =6.626×10-25J =6.02×1023×6.626×10-34J·s×10°s1 E=h.v =6.626×10-2J 400nm 500 600 700
The electromagnetic spectrum The visible light --- electromagnetic radiation E = ⋅ h ν Photon 34 9 1 25 6.626 10 10 6.626 10 E J h s s J ν − − − = ⋅ × ⋅ × = × = 23 34 9 1 2 6.02 10 6.626 10 10 6.626 10 Emole J A J h s s ν − − − = ⋅ ⋅ = × × × ⋅ × = ×
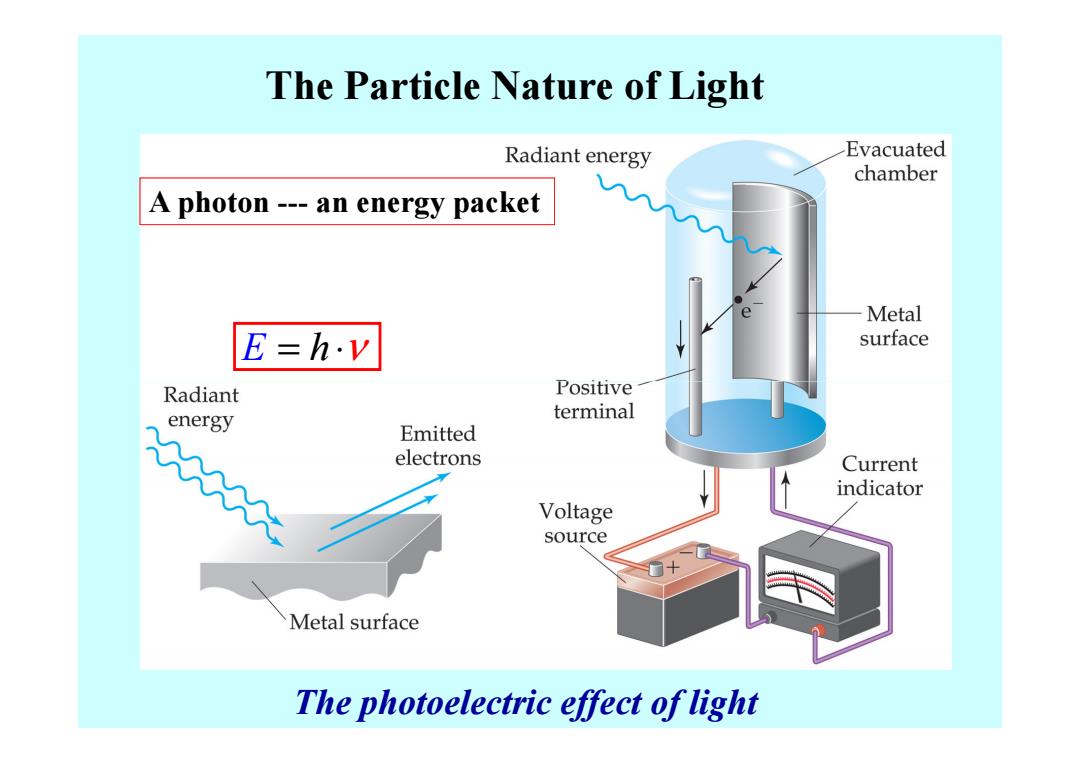
The Particle Nature of Light Radiant energy Evacuated chamber A photon---an energy packet Metal E=h.v surface Radiant Positive energy terminal Emitted electrons Current indicator Voltage source Metal surface The photoelectric effect of light
The Particle Nature of Light E = ⋅ h ν A photon --- an energy packet The photoelectric effect of light