
(1)纯 n 级反应 若 const ,则 A,0 E,0 F,0 = = = f c e c a c n n k c t c r B B B d 1 d = = ★ n = 1 k t c c 1 B B,0 B ln = exp( ) B B,0 B,0 1 B 1 B c c e c k t k t 或 = = 2 B,0 1 c c 当 B = 时, B 1 B 1 ln 2 0.693 2 1 k k t t = = − = − 特征:①k1与浓度单位无关, 单位:(时间) -1 ; ②lncB 与t 呈线性关系,斜率 = νBk1 ; ③t1/2与νBk1有关,与cB,0无关
(1)纯 n 级反应 若 const ,则 A,0 E,0 F,0 = = = f c e c a c n n k c t c r B B B d 1 d = = ★ n = 1 k t c c 1 B B,0 B ln = exp( ) B B,0 B,0 1 B 1 B c c e c k t k t 或 = = 2 B,0 1 c c 当 B = 时, B 1 B 1 ln 2 0.693 2 1 k k t t = = − = − 特征:①k1与浓度单位无关, 单位:(时间) -1 ; ②lncB 与t 呈线性关系,斜率 = νBk1 ; ③t1/2与νBk1有关,与cB,0无关
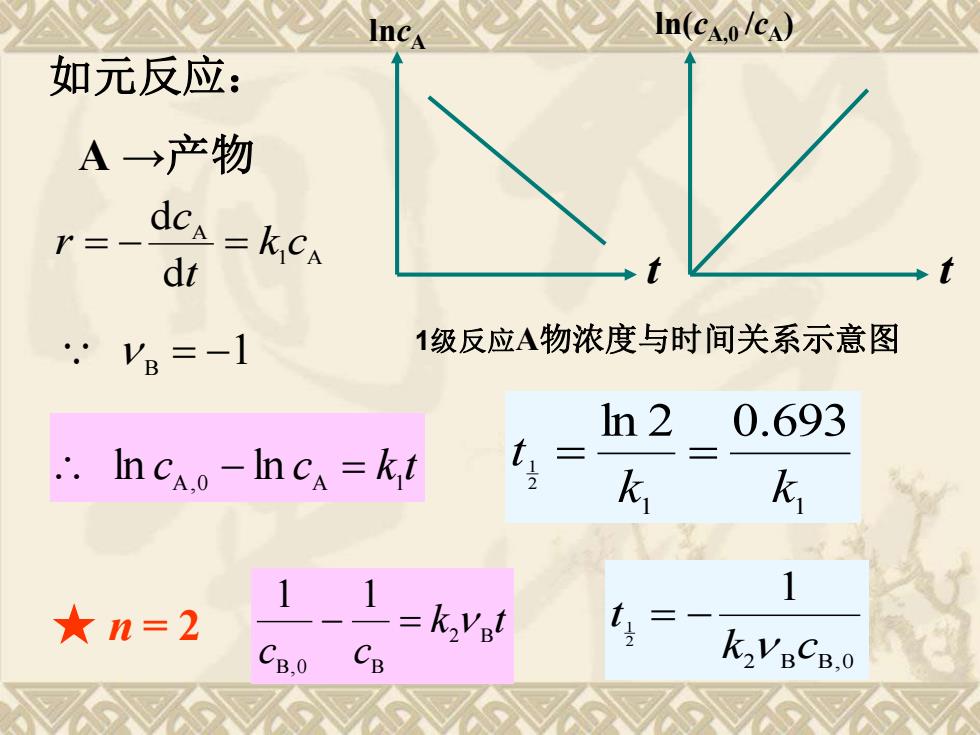
★ n = 2 k t c c 2 B B,0 B 1 1 − = ln(cA,0 /cA) t 如元反应: A →产物 1 A A d d k c t c r = − = c c k t A,0 A 1 ln − ln = 1 1 ln 2 0.693 2 1 k k t = = B = −1 lncA t 1级反应A物浓度与时间关系示意图 2 B B,0 1 2 1 k c t = −
★ n = 2 k t c c 2 B B,0 B 1 1 − = ln(cA,0 /cA) t 如元反应: A →产物 1 A A d d k c t c r = − = c c k t A,0 A 1 ln − ln = 1 1 ln 2 0.693 2 1 k k t = = B = −1 lncA t 1级反应A物浓度与时间关系示意图 2 B B,0 1 2 1 k c t = −