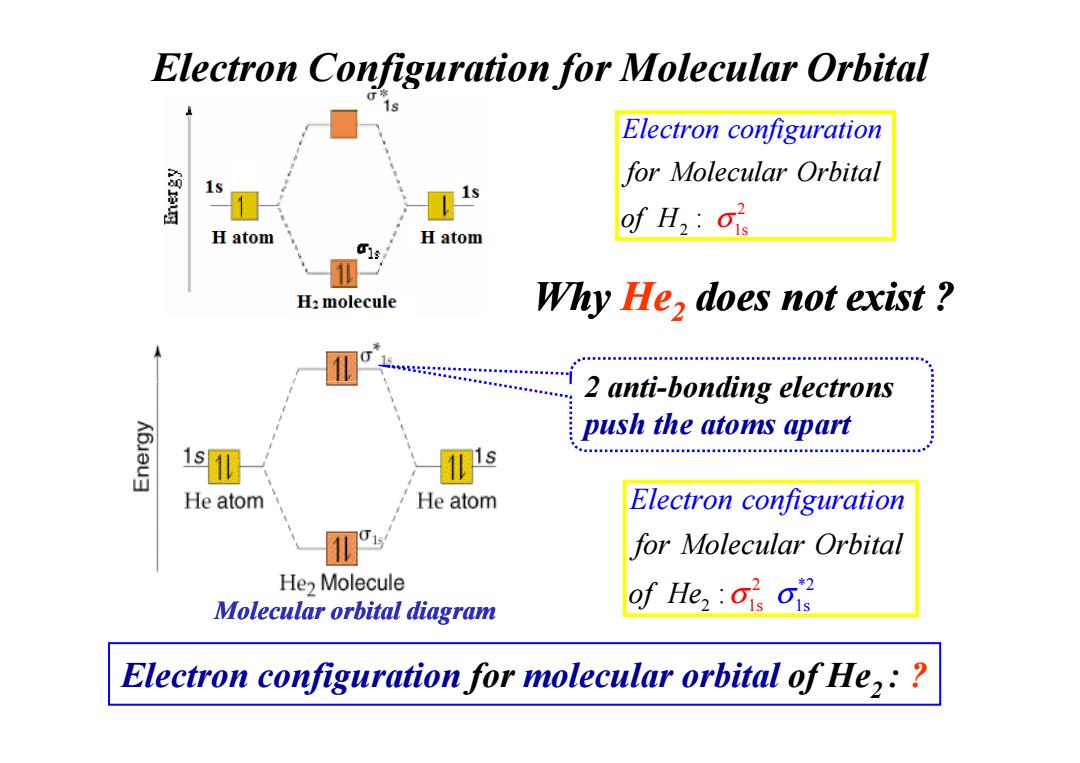
Electron Configuration for Molecular Orbital Electron configuration for Molecular Orbital 1s of H2:ois H atom H atom H:molecule Why He,does not exist 2 anti-bonding electrons K6Jau3 push the atoms apart 1 s He atom He atom Electron configuration for Molecular Orbital He2 Molecule Molecular orbital diagram of Hes Electron configuration for molecular orbital of He,:
Why He2 does not exist ? Electron Configuration for Molecular Orbital 2 anti-bonding electrons 2 2 1s : for Molecular Orbit E a lectron configuration l of H σ 2 anti-bonding electrons push the atoms apart 1 *2 2 1s 2 s : for Molecular Orbit Electron configuratio l f n a o He σ σ Molecular orbital diagram Electron configuration for molecular orbital of He2 : ?
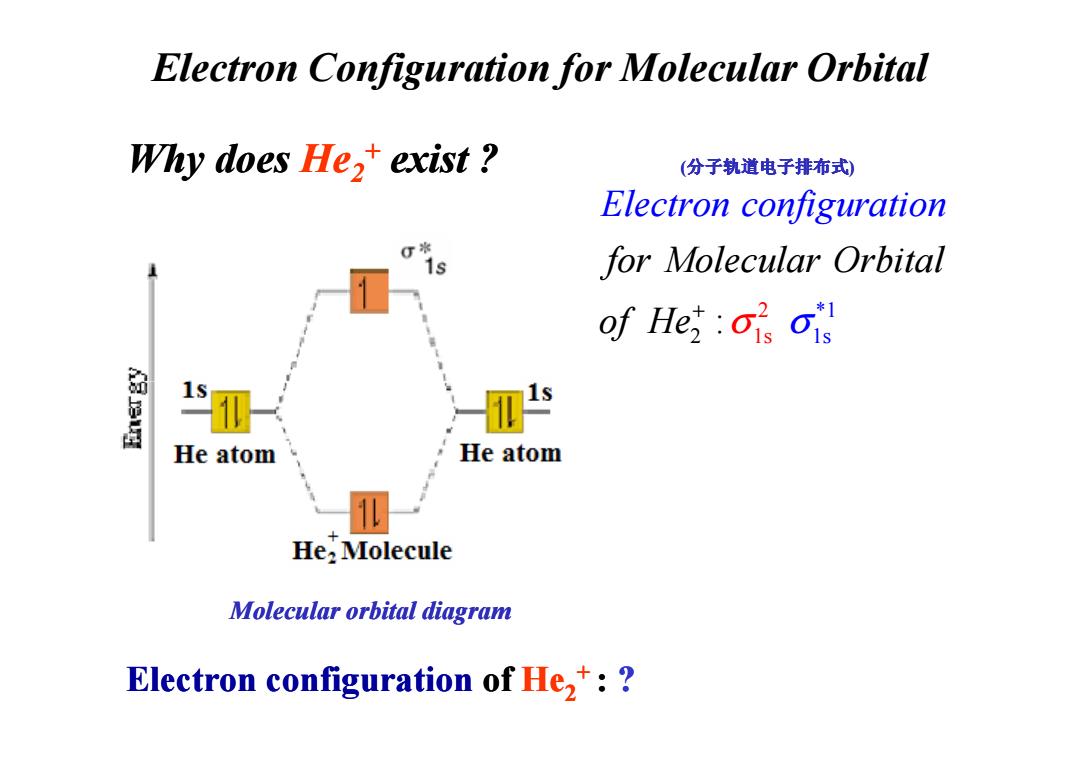
Electron Configuration for Molecular Orbital Why does He,+exist? (分子轨道电子排布式) Electron configuration for Molecular Orbital of He :OisOis He atom He atom He:Molecule Molecular orbital diagram Electron configuration of He,:
Why does He2+ exist ? Electron Configuration for Molecular Orbital 2 1 *1 1s 2 s : for Molecular Orbital of H Electron configuration e σ σ + (分子轨道电子排布式) Electron configuration of He2+ : ? Molecular orbital diagram
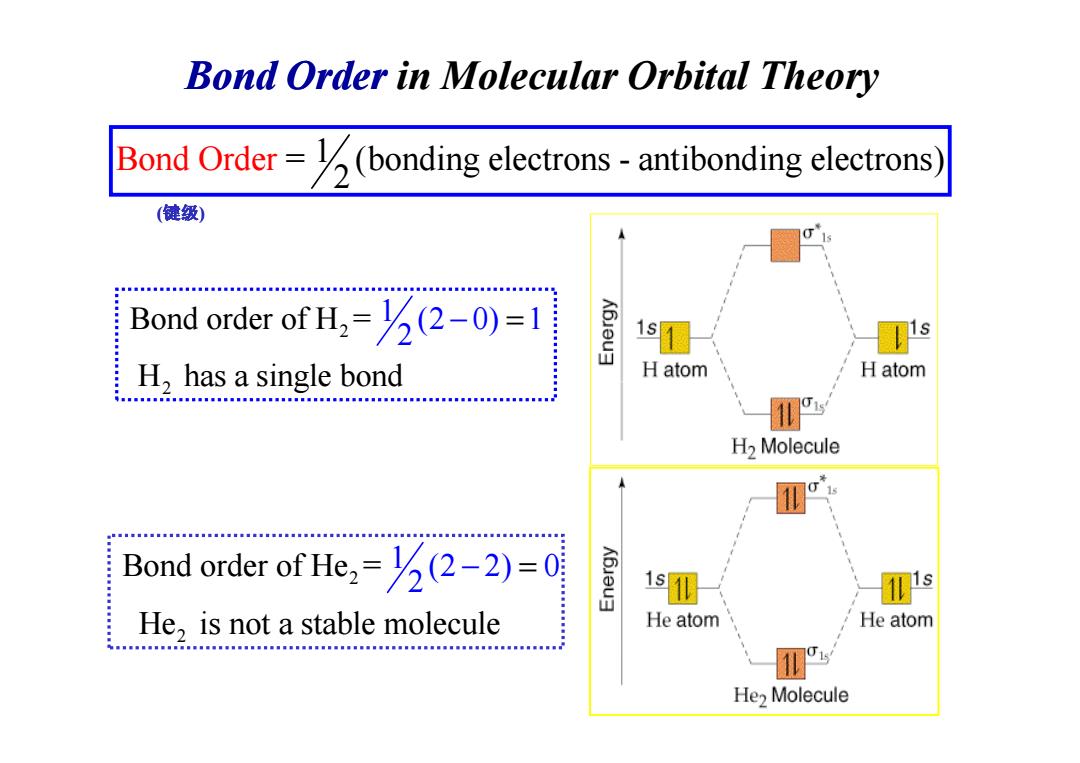
Bond Order in Molecular Orbital Theory Bond Order=(bonding electrons-antibonding electrons (健级) Bond order of H=(2-0)=1 1s s H,has a single bond H atom H atom HHEH.HEM..MNMMM.MN.MMM3an...... H2 Molecule 11 Bond order of He=(2-2)=0 He,is not a stable molecule He atom He atom He>Molecule
Bond Order in Molecular Orbital Theory Bond Ord = (bonding electrons - antibonding 1 electr 2 er ons) 2 2 1 Bond order of H = (2 0) 1 H has a singl 2 e bond − = (键级) 2 H has a single bond 2 2 Bond order of He = He is not a stable 1 (2 m 2) 0 2 olecule − =