
encode subunits of the the rapid delayed rectifier potassium current(Ikr),whereas KCNOI and KCNEI (mink)encode subunits of the slow delayed rectifier potassium current (Iks).KCNJ2 encodes an inwardly rectifying potassium current (Ikir).In contrast,gain of function mutations in the sodium channel gene (SCN5A)or calcium channel gene (CACNAIc)cause increases in inward plateau current and are responsible for LQT subtypes 3 and 8,respectively Molecular genetic studies have identified the reason why congenital and acquired cases of torsades de pointes can be so strikingly similar.The potassium channel Ikn (encoded by HERG)is blocked or modified by many drugs(eg,quinidine,sotalol)or electrolyte abnormalities (hypokalemia,hypomagnesemia,hypocalcemia)that also produce torsades de pointes.Thus,the identification of the precise molecular mechanisms underlying various forms of the LOT syndromes now raises the possibility that specific therapies may be developed for individuals with defined molecular abnormalities.Indeed,preliminary reports suggest that the sodium channel blocker mexiletine can correct the clinical manifestations of congenital LOT subtype 3 syndrome.It is likely that torsade de pointes originates from triggered upstrokes arising from early afterdepolarizations (Figure 5).Thus,therapy is directed at correcting hypokalemia,eliminating triggered upstrokes (eg,by using blockers or magnesium),or shortening the action potential (eg,by increasing heart rate with isoproterenol or pacing)or all of these. The molecular basis of several other congenital cardiac arrhythmias associated with sudden death has also recently been identified.Three forms of short QT syndrome have been identified that are linked to gain of function mutations in three different potassium channel genes (KCNH2,KCNOI,and KCNJ2).Catecholaminergic polymorphic ventricular tachycardia,a disease that is characterized by stress-or emotion-induced syncope,can be caused by genetic mutations in two different proteins in the sarcoplasmic reticulum that control intracellular calcium homeostasis. Mutations in two different ion channel genes (HCN4 and SCN5A)have been linked to congenital forms of sick sinus syndrome.The Brugada syndrome,which is characterized by ventricular fibrillation associated with persistent ST-segment elevation,and progressive cardiac conduction disorder (PCCD),characterized by impaired conduction in the His-Purkinje system and right or left bundle block leading to complete atrioventricular block,have both been linked to several loss-of-function mutations in the sodium channel gene,SCN54.At least one form of familial atrial fibrillation is caused by a gain-of-function mutation in the potassium channel gene, KCNO1. 11
11 encode subunits of the the rapid delayed rectifier potassium current (IKr), whereas KCNQ1 and KCNE1 (minK) encode subunits of the slow delayed rectifier potassium current (IKs). KCNJ2 encodes an inwardly rectifying potassium current (IKir). In contrast, gain of function mutations in the sodium channel gene (SCN5A) or calcium channel gene (CACNA1c) cause increases in inward plateau current and are responsible for LQT subtypes 3 and 8, respectively. Molecular genetic studies have identified the reason why congenital and acquired cases of torsades de pointes can be so strikingly similar. The potassium channel IKr (encoded by HERG) is blocked or modified by many drugs (eg, quinidine, sotalol) or electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia) that also produce torsades de pointes. Thus, the identification of the precise molecular mechanisms underlying various forms of the LQT syndromes now raises the possibility that specific therapies may be developed for individuals with defined molecular abnormalities. Indeed, preliminary reports suggest that the sodium channel blocker mexiletine can correct the clinical manifestations of congenital LQT subtype 3 syndrome. It is likely that torsade de pointes originates from triggered upstrokes arising from early afterdepolarizations (Figure 5). Thus, therapy is directed at correcting hypokalemia, eliminating triggered upstrokes (eg, by using blockers or magnesium), or shortening the action potential (eg, by increasing heart rate with isoproterenol or pacing) or all of these. The molecular basis of several other congenital cardiac arrhythmias associated with sudden death has also recently been identified. Three forms of short QT syndrome have been identified that are linked to gain of function mutations in three different potassium channel genes (KCNH2, KCNQ1, and KCNJ2). Catecholaminergic polymorphic ventricular tachycardia, a disease that is characterized by stress- or emotion-induced syncope, can be caused by genetic mutations in two different proteins in the sarcoplasmic reticulum that control intracellular calcium homeostasis. Mutations in two different ion channel genes (HCN4 and SCN5A) have been linked to congenital forms of sick sinus syndrome. The Brugada syndrome, which is characterized by ventricular fibrillation associated with persistent ST-segment elevation, and progressive cardiac conduction disorder (PCCD), characterized by impaired conduction in the His-Purkinje system and right or left bundle block leading to complete atrioventricular block, have both been linked to several loss-of-function mutations in the sodium channel gene, SCN5A. At least one form of familial atrial fibrillation is caused by a gain-of-function mutation in the potassium channel gene, KCNQ1
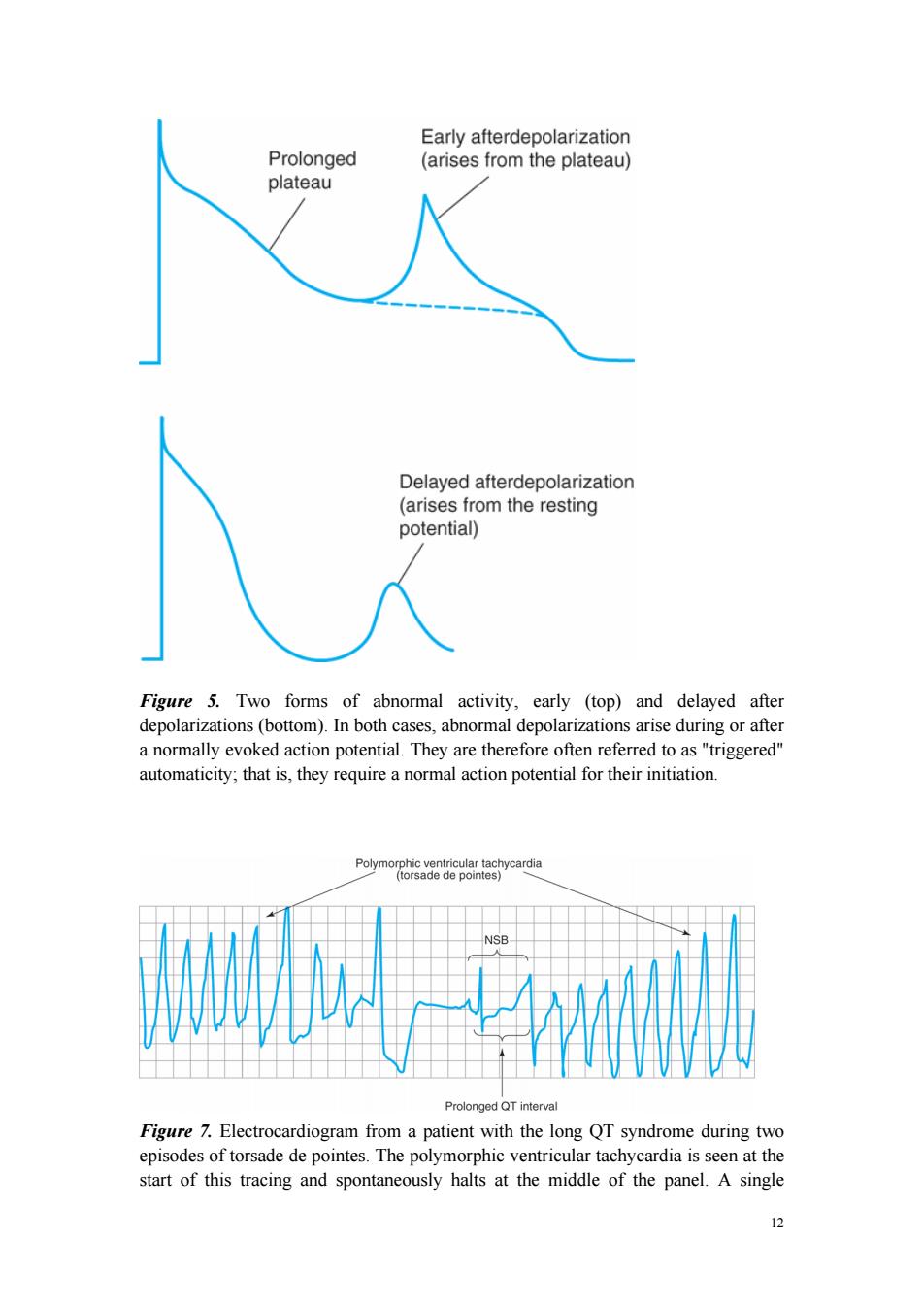
Early afterdepolarization Prolonged (arises from the plateau) plateau Delayed afterdepolarization (arises from the resting potential)) Figure 5.Two forms of abnormal activity,early (top)and delayed after depolarizations(bottom).In both cases,abnormal depolarizations arise during or after a normally evoked action potential.They are therefore often referred to as "triggered" automaticity;that is,they require a normal action potential for their initiation. Polymorphic ventricular tachycardia (torsade de pointes) NSB Prolonged QT interval Figure 7.Electrocardiogram from a patient with the long QT syndrome during two episodes of torsade de pointes.The polymorphic ventricular tachycardia is seen at the start of this tracing and spontaneously halts at the middle of the panel.A single 12
12 Figure 5. Two forms of abnormal activity, early (top) and delayed after depolarizations (bottom). In both cases, abnormal depolarizations arise during or after a normally evoked action potential. They are therefore often referred to as "triggered" automaticity; that is, they require a normal action potential for their initiation. Figure 7. Electrocardiogram from a patient with the long QT syndrome during two episodes of torsade de pointes. The polymorphic ventricular tachycardia is seen at the start of this tracing and spontaneously halts at the middle of the panel. A single

normal sinus beat(NSB)with an extremely prolonged QT interval follows,succeeded immediately by another episode of ventricular tachycardia of the torsade type.The usual symptoms would be dizziness or transient loss of consciousness. Disturbances of Impulse Conduction Severely depressed conduction may result in simple block,eg,atrioventricular nodal block or bundle branch block.Because parasympathetic control of atrioventricular conduction is significant,partial atrioventricular block is sometimes relieved by atropine.Another common abnormality of conduction is reentry (also known as "circus movement"),in which one impulse reenters and excites areas of the heart more than once(Figure 6).The path of the reentering impulse may be confined to very small areas,eg,within or near the atrioventricular node,or it may involve large portions of the atrial or ventricular walls.Some forms of reentry are strictly anatomically determined;for example,in the Wolff-Parkinson-White syndrome,the reentry circuit consists of atrial tissue,the Av node,ventricular tissue,and an accessory atrioventricular connection (a "bypass tract").In other cases (eg,atrial or ventricular fibrillation),multiple reentry circuits,determined by the properties of the cardiac tissue,may meander through the heart in apparently random paths. Furthermore,the circulating impulse often gives off "daughter impulses"that can spread to the rest of the heart.Depending on how many round trips through the pathway the impulse makes before dying out,the arrhythmia may be manifest as one or a few extra beats or as a sustained tachycardia.In order for reentry to occur,three conditions must coexist,as indicated in Figure 6:(1)There must be an obstacle (anatomic or physiologic)to homogeneous conduction,thus establishing a circuit around which the reentrant wavefront can propagate;(2)there must be unidirectional block at some point in the circuit,ie conduction must die out in one direction but continue in the opposite direction (as shown in Figure 6,the impulse can gradually decrease as it invades progressively more depolarized tissue until it finally blocks a process known as decremental conduction);and (3)conduction time around the circuit must be long enough so that the retrograde impulse does not enter refractory tissue as it travels around the obstacle,ie the conduction time must exceed the effective refractory period.Importantly,reentry depends on conduction that has been depressed by some critical amount,usually as a result of injury or ischemia.If conduction velocity is too slow,bidirectional block rather than unidirectional block occurs;if the reentering impulse is too weak,conduction may fail,or the impulse may arrive so late that it collides with the next regular impulse.On the other hand,if conduction is too rapid,ie almost normal,bidirectional conduction rather than unidirectional block will occur.Even in the presence of unidirectional block,if the impulse travels around the obstacle too rapidly,it will reach tissue that is still refractory. Slowing of conduction may be due to depression of sodium current,depression of calcium current(the latter especially in the atrioventricular node),or both.Drugs that 3
13 normal sinus beat (NSB) with an extremely prolonged QT interval follows, succeeded immediately by another episode of ventricular tachycardia of the torsade type. The usual symptoms would be dizziness or transient loss of consciousness. Disturbances of Impulse Conduction Severely depressed conduction may result in simple block, eg, atrioventricular nodal block or bundle branch block. Because parasympathetic control of atrioventricular conduction is significant, partial atrioventricular block is sometimes relieved by atropine. Another common abnormality of conduction is reentry (also known as "circus movement"), in which one impulse reenters and excites areas of the heart more than once (Figure 6). The path of the reentering impulse may be confined to very small areas, eg, within or near the atrioventricular node, or it may involve large portions of the atrial or ventricular walls. Some forms of reentry are strictly anatomically determined; for example, in the Wolff-Parkinson-White syndrome, the reentry circuit consists of atrial tissue, the AV node, ventricular tissue, and an accessory atrioventricular connection (a "bypass tract"). In other cases (eg, atrial or ventricular fibrillation), multiple reentry circuits, determined by the properties of the cardiac tissue, may meander through the heart in apparently random paths. Furthermore, the circulating impulse often gives off "daughter impulses" that can spread to the rest of the heart. Depending on how many round trips through the pathway the impulse makes before dying out, the arrhythmia may be manifest as one or a few extra beats or as a sustained tachycardia. In order for reentry to occur, three conditions must coexist, as indicated in Figure 6: (1) There must be an obstacle (anatomic or physiologic) to homogeneous conduction, thus establishing a circuit around which the reentrant wavefront can propagate; (2) there must be unidirectional block at some point in the circuit, ie conduction must die out in one direction but continue in the opposite direction (as shown in Figure 6, the impulse can gradually decrease as it invades progressively more depolarized tissue until it finally blocks a process known as decremental conduction); and (3) conduction time around the circuit must be long enough so that the retrograde impulse does not enter refractory tissue as it travels around the obstacle, ie the conduction time must exceed the effective refractory period. Importantly, reentry depends on conduction that has been depressed by some critical amount, usually as a result of injury or ischemia. If conduction velocity is too slow, bidirectional block rather than unidirectional block occurs; if the reentering impulse is too weak, conduction may fail, or the impulse may arrive so late that it collides with the next regular impulse. On the other hand, if conduction is too rapid, ie almost normal, bidirectional conduction rather than unidirectional block will occur. Even in the presence of unidirectional block, if the impulse travels around the obstacle too rapidly, it will reach tissue that is still refractory. Slowing of conduction may be due to depression of sodium current, depression of calcium current (the latter especially in the atrioventricular node), or both. Drugs that