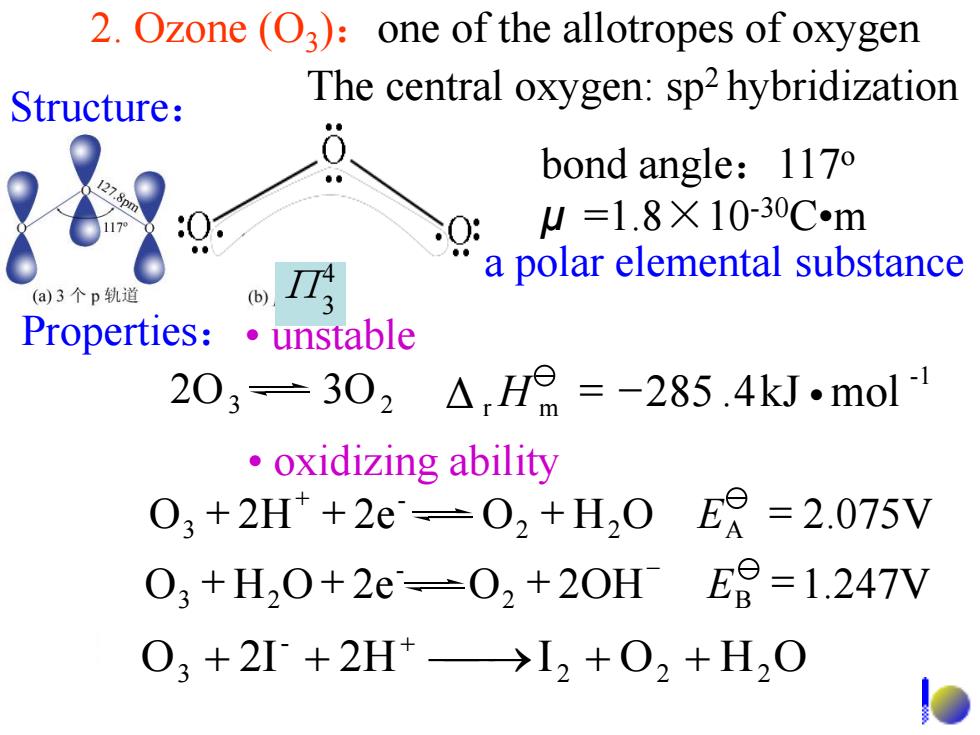
2.Ozone (O3):one of the allotropes of oxygen Structure: The central oxygen:sp2 hybridization bond angle:1170 SO: W=1.8×10-30Cm a polar elemental substance (a)3个p轨道 Properties:unstable 203=302△H=-285.4kJ.mol ·oxidizing ability 03+2H+2e-02+H,0ER=2.075V 03+H,0+2e-02+20HE9=-1.247V 03+2I+2H+→I2+02+H2O
2. Ozone (O3 ):one of the allotropes of oxygen O 2I 2H I 2 O2 H2 O - 3 + + + + + Structure: The central oxygen: sp2 hybridization bond angle:117o μ =1.8×10-30C•m Properties:• unstable • oxidizing ability a polar elemental substance 2O3 3O2 -1 Δ r H m = -285 .4kJ •mol O 2H 2e O2 H2O A 2.075V - 3 + + + = + E O H O 2e O2 2OH B 1.247V - 3 2 + + + = - E 4 Π3
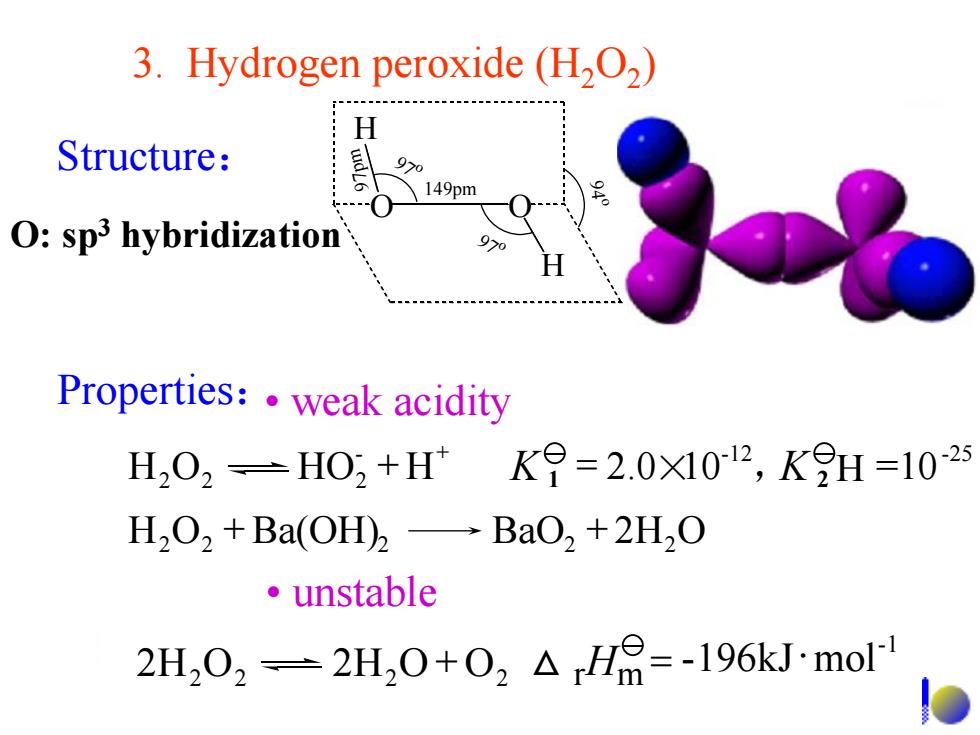
3.Hydrogen peroxide(H2O2) H Structure: 9>0 149pm O:sp3 hybridization 9>0 Properties:.weak acidity H,02=H0,+HK9=2.0X1012,KH=1025 H2O2+Ba(OH)2-BaO2+2H2O ·unstable 2H202=2H,0+02△H=-196 kJ.mol
3. Hydrogen peroxide (H2O2 ) • unstable O O H H 149pm 97 o 97 o 97pm 94 o Properties:• weak acidity -1 2H2O2 2H2O + O2 = -196kJmol △ rHm H2O2 + Ba(OH)2 BaO2 + 2H2O H O HO H 2.0 10 , =10 - -12 -25 2 2 2 + = × + K 1 K 2 Structure: O: sp3 hybridization
·redox property in acidic 020.6945VH021.763VH0 solution: H202+2Fe2++2Ht→2Fe3++2H,0 oxidant 4H202+PbS(s,黑)→PbS04(s,白+4H2O reducer 5H,02+2MnO4+6H→2Mn2++502+8H,0 in basic H02+H,0+2e=3OH,E8=0.867V solution 3H,O,+2Cr(OH)a +20H>2CrO+8H,O we can see the oxidation ability of H2O2 is strong and reduction ability is weak;So,it can be used as bactericide
we can see the oxidation ability of H2O2 is strong and reduction ability is weak;So, it can be used as bactericide. 3H O 2Cr(OH) 2OH 2CrO 8H2 O 2- 4 - - 2 2 + 4 + + H O 2Fe 2H 2Fe 2H2 O 2 3 2 2 + + + + + + in acidic solution: O2 0.6945V H2 O2 1.763V H2 O • redox property HO H O 2e 3OH , 0.867V - - 2 - 2 + + E = B 5H O 2MnO 6H 2Mn 5O2 8H2 O - 2 2 2 + 4 + + + + + 4H2 O2 + PbS(s,黑) PbSO4 (s,白) + 4H2 O in basic solution: oxidant reducer

16.3 Sulfur and related compounds 1.Elemental sulfur:a few allotropic forms rhombic sulfur monoclinic sulfur plastic sulfur (斜方硫) (单斜硫) (弹性硫) density/g'cm3 2.06 1.99 color yellow buff fast cooling of liquid (浅黄) sulfur heated above stability <94.5℃ >94.5℃ 190℃ Structure: a puckered Sa ring structure S:sp3 hybridization for both斜方硫and单斜硫
rhombic sulfur monoclinic sulfur plastic sulfur (斜方硫) (单斜硫 ) (弹性硫) density/gcm-3 2.06 1.99 color yellow buff fast cooling of liquid (浅黄) sulfur heated above stability < 94.5℃ > 94.5℃ 190℃ 1. Elemental sulfur: a few allotropic forms 16.3 Sulfur and related compounds Structure: S:sp3 hybridization for both 斜方硫 and 单斜硫 a puckered S8 ring structure
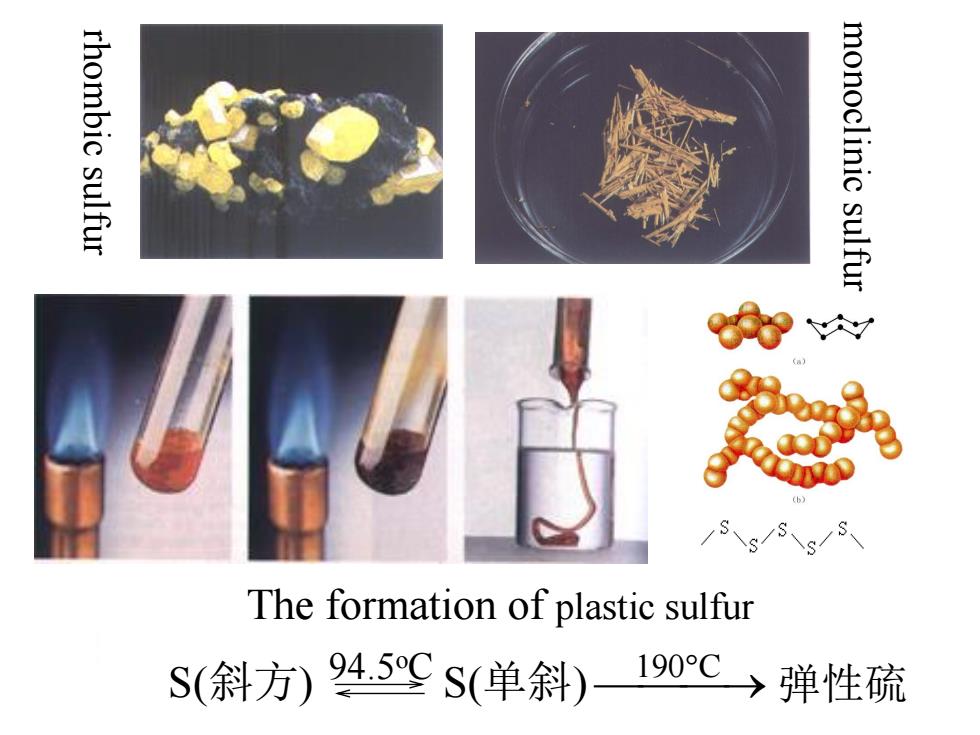
rhombic sulfur monoclinic sulfur The formation of plastic sulfur S(斜方)945SS(单斜)190℃→弹性硫
rhombic sulfur monoclinic sulfur The formation of plastic sulfur S(斜方) 94.5 S(单斜) 190 C 弹性硫 oC