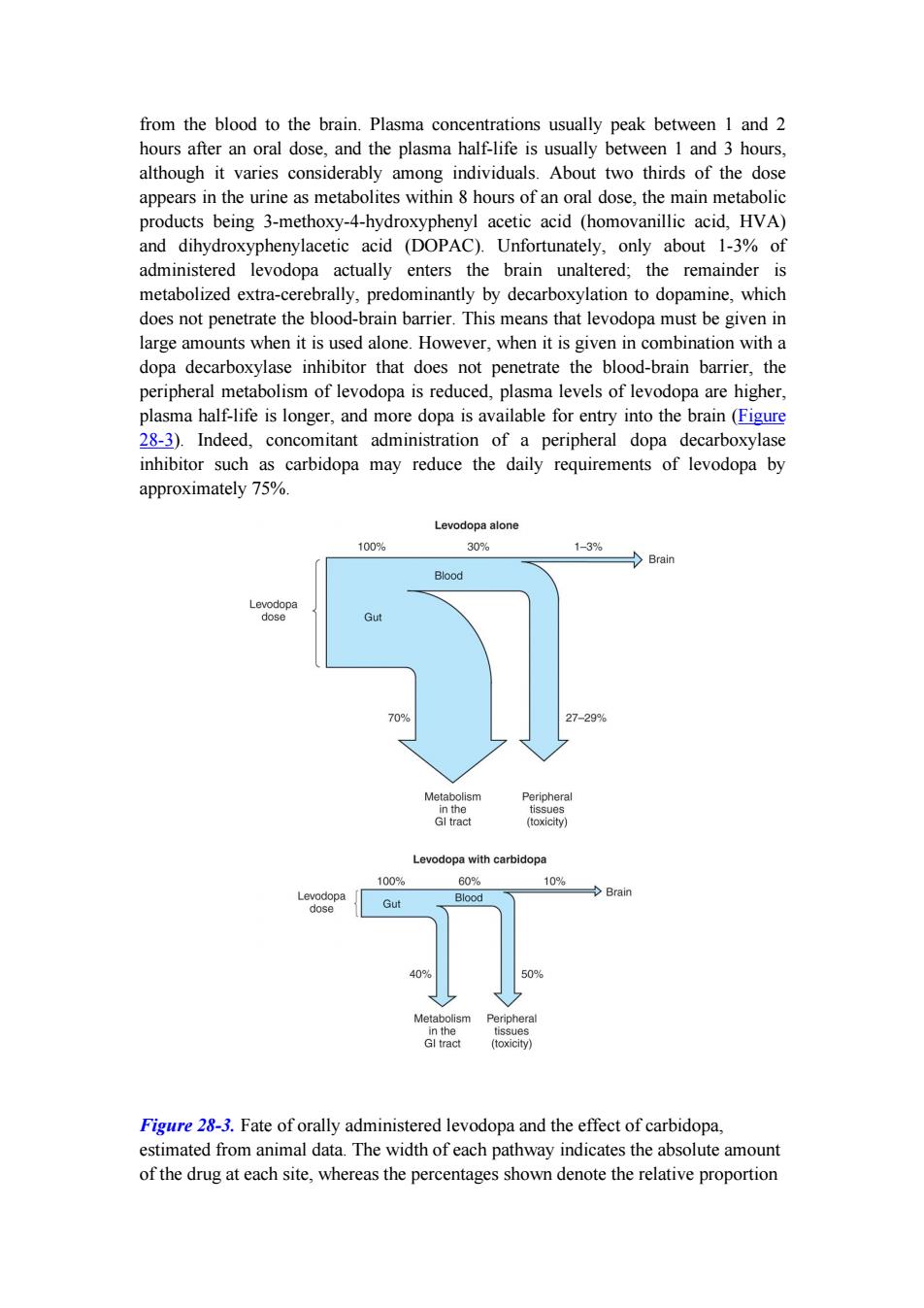
from the blood to the brain.Plasma concentrations usually peak between 1 and 2 hours after an oral dose,and the plasma half-life is usually between 1 and 3 hours, although it varies considerably among individuals.About two thirds of the dose appears in the urine as metabolites within 8 hours of an oral dose,the main metabolic products being 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid,HVA) and dihydroxyphenylacetic acid (DOPAC).Unfortunately,only about 1-3%of administered levodopa actually enters the brain unaltered;the remainder is metabolized extra-cerebrally,predominantly by decarboxylation to dopamine,which does not penetrate the blood-brain barrier.This means that levodopa must be given in large amounts when it is used alone.However,when it is given in combination with a dopa decarboxylase inhibitor that does not penetrate the blood-brain barrier,the peripheral metabolism of levodopa is reduced,plasma levels of levodopa are higher, plasma half-life is longer,and more dopa is available for entry into the brain(Figure 28-3).Indeed,concomitant administration of a peripheral dopa decarboxylase inhibitor such as carbidopa may reduce the daily requirements of levodopa by approximately 75%. Levodopa alone 100% 30% 1-3% Brain Blood Levodopa dose Gut 70% 27-29% Metabolism Peripheral in the tissues GI tract (toxicity) Levodopa with carbidopa 100% 60% 10% Levodopa Gut Blood Brain dase 409 50% Metabolism Peripheral in the tissues Gl tract (toxicity) Figure 28-3.Fate of orally administered levodopa and the effect of carbidopa, estimated from animal data.The width of each pathway indicates the absolute amount of the drug at each site,whereas the percentages shown denote the relative proportion
from the blood to the brain. Plasma concentrations usually peak between 1 and 2 hours after an oral dose, and the plasma half-life is usually between 1 and 3 hours, although it varies considerably among individuals. About two thirds of the dose appears in the urine as metabolites within 8 hours of an oral dose, the main metabolic products being 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid, HVA) and dihydroxyphenylacetic acid (DOPAC). Unfortunately, only about 1-3% of administered levodopa actually enters the brain unaltered; the remainder is metabolized extra-cerebrally, predominantly by decarboxylation to dopamine, which does not penetrate the blood-brain barrier. This means that levodopa must be given in large amounts when it is used alone. However, when it is given in combination with a dopa decarboxylase inhibitor that does not penetrate the blood-brain barrier, the peripheral metabolism of levodopa is reduced, plasma levels of levodopa are higher, plasma half-life is longer, and more dopa is available for entry into the brain (Figure 28-3). Indeed, concomitant administration of a peripheral dopa decarboxylase inhibitor such as carbidopa may reduce the daily requirements of levodopa by approximately 75%. Figure 28-3. Fate of orally administered levodopa and the effect of carbidopa, estimated from animal data. The width of each pathway indicates the absolute amount of the drug at each site, whereas the percentages shown denote the relative proportion

of the administered dose.The benefits of coadministration of carbidopa include reduction of the amount of levodopa diverted to peripheral tissues and an increase in the fraction of the dose that reaches the brain.(GI,gastrointestinal.)(Data from Nutt JG,Fellman JH:Pharmacokinetics of levodopa.Clin Neuropharmacol 1984;7:35.) Clinical Use The best results of levodopa treatment are obtained in the first few years of treatment. This is sometimes because the daily dose of levodopa must be reduced over time to avoid side effects at doses that were well tolerated at the outset.Some patients also become less responsive to levodopa,so that previously effective doses eventually fail to produce any therapeutic benefit.Responsiveness to levodopa may ultimately be lost completely,perhaps because of the disappearance of dopaminergic nigrostriatal nerve terminals or some pathologic process directly involving the striatal dopamine receptors.For such reasons,the benefits of levodopa treatment often begin to diminish after about 3 or 4 years of therapy regardless of the initial therapeutic response. Although levodopa therapy does not stop the progression of parkinsonism,its early initiation lowers the mortality rate.However,long-term therapy may lead to a number of problems in management such as the on-off phenomenon discussed below.The most appropriate time to introduce levodopa therapy must therefore be determined individually. When levodopa is used,it is generally given in combination with carbidopa(Figure 28-2),a peripheral dopa decarboxylase inhibitor,which reduces peripheral conversion to dopamine.Sinemet treatment is started with a small dose,eg,Sinemet-25/100 (carbidopa 25 mg,levodopa 100 mg)three times daily,and gradually increased.It should be taken 30-60 minutes before meals.Most patients ultimately require Sinemet-25/250(carbidopa 25 mg,levodopa 250 mg)three or four times daily.It is generally preferable to keep treatment with this agent at a low level (eg, Sinemet-25/100 three times daily)and to increase dopaminergic therapy by the addition of a dopamine agonist,if necessary,to reduce the risk of development of response fluctuations.A controlled-release formulation of Sinemet is available and may be helpful in patients with established response fluctuations or as a means of reducing dosing frequency.A commercially available combination (Stalevo)of levodopa,carbidopa,and a catechol-O-methyltransferase (COMT)inhibitor (entacapone)is discussed in a later section. Levodopa can ameliorate all of the clinical features of parkinsonism,but it is particularly effective in relieving bradykinesia and any disabilities resulting from it. When it is first introduced,about one third of patients respond very well and one third less well.Most of the remainder either are unable to tolerate the medication or simply do not respond at all. Adverse Effects
of the administered dose. The benefits of coadministration of carbidopa include reduction of the amount of levodopa diverted to peripheral tissues and an increase in the fraction of the dose that reaches the brain. (GI, gastrointestinal.) (Data from Nutt JG, Fellman JH: Pharmacokinetics of levodopa. Clin Neuropharmacol 1984;7:35.) Clinical Use The best results of levodopa treatment are obtained in the first few years of treatment. This is sometimes because the daily dose of levodopa must be reduced over time to avoid side effects at doses that were well tolerated at the outset. Some patients also become less responsive to levodopa, so that previously effective doses eventually fail to produce any therapeutic benefit. Responsiveness to levodopa may ultimately be lost completely, perhaps because of the disappearance of dopaminergic nigrostriatal nerve terminals or some pathologic process directly involving the striatal dopamine receptors. For such reasons, the benefits of levodopa treatment often begin to diminish after about 3 or 4 years of therapy regardless of the initial therapeutic response. Although levodopa therapy does not stop the progression of parkinsonism, its early initiation lowers the mortality rate. However, long-term therapy may lead to a number of problems in management such as the on-off phenomenon discussed below. The most appropriate time to introduce levodopa therapy must therefore be determined individually. When levodopa is used, it is generally given in combination with carbidopa (Figure 28-2), a peripheral dopa decarboxylase inhibitor, which reduces peripheral conversion to dopamine. Sinemet treatment is started with a small dose, eg, Sinemet-25/100 (carbidopa 25 mg, levodopa 100 mg) three times daily, and gradually increased. It should be taken 30-60 minutes before meals. Most patients ultimately require Sinemet-25/250 (carbidopa 25 mg, levodopa 250 mg) three or four times daily. It is generally preferable to keep treatment with this agent at a low level (eg, Sinemet-25/100 three times daily) and to increase dopaminergic therapy by the addition of a dopamine agonist, if necessary, to reduce the risk of development of response fluctuations. A controlled-release formulation of Sinemet is available and may be helpful in patients with established response fluctuations or as a means of reducing dosing frequency. A commercially available combination (Stalevo) of levodopa, carbidopa, and a catechol-O-methyltransferase (COMT) inhibitor (entacapone) is discussed in a later section. Levodopa can ameliorate all of the clinical features of parkinsonism, but it is particularly effective in relieving bradykinesia and any disabilities resulting from it. When it is first introduced, about one third of patients respond very well and one third less well. Most of the remainder either are unable to tolerate the medication or simply do not respond at all. Adverse Effects

A.GASTROINTESTINAL EFFECTS When levodopa is given without a peripheral decarboxylase inhibitor,anorexia and nausea and vomiting occur in about 80%of patients.These adverse effects can be minimized by taking the drug in divided doses,with or immediately after meals,and by increasing the total daily dose very slowly;antacids taken 30-60 minutes before levodopa may also be beneficial.The vomiting has been attributed to stimulation of the chemoreceptor trigger zone located in the brainstem but outside the blood-brain barrier.Fortunately,tolerance to this emetic effect develops in many patients. Antiemetics such as phenothiazines should be avoided because they reduce the antiparkinsonism effects of levodopa and may exacerbate the disease. When levodopa is given in combination with carbidopa,adverse gastrointestinal effects are much less frequent and troublesome,occurring in less than 20%of cases, so that patients can tolerate proportionately higher doses. B.CARDIOVASCULAR EFFECTS A variety of cardiac arrhythmias have been described in patients receiving levodopa, including tachycardia,ventricular extrasystoles and,rarely,atrial fibrillation.This effect has been attributed to increased catecholamine formation peripherally.The incidence of such arrhythmias is low,even in the presence of established cardiac disease,and may be reduced still further if the levodopa is taken in combination with a peripheral decarboxylase inhibitor. Postural hypotension is common,but often asymptomatic,and tends to diminish with continuing treatment.Hypertension may also occur,especially in the presence of nonselective monoamine oxidase inhibitors or sympathomimetics or when massive doses of levodopa are being taken. C.DYSKINESIAS Dyskinesias occur in up to 80%of patients receiving levodopa therapy for long periods.The form and nature of dopa dyskinesias vary widely among patients but tend to remain constant in character in individual patients.Choreoathetosis of the face and distal extremities is the most common presentation.The development of dyskinesias is dose-related.but there is considerable individual variation in the dose required to produce them. D.BEHAVIORAL EFFECTS A wide variety of adverse mental effects have been reported,including depression, anxiety,agitation,insomnia,somnolence,confusion,delusions,hallucinations, nightmares,euphoria,and other changes in mood or personality.Such adverse effects are more common in patients taking levodopa in combination with a decarboxylase inhibitor rather than levodopa alone,presumably because higher levels are reached in the brain.They may be precipitated by intercurrent illness or operation.It may be
A. GASTROINTESTINAL EFFECTS When levodopa is given without a peripheral decarboxylase inhibitor, anorexia and nausea and vomiting occur in about 80% of patients. These adverse effects can be minimized by taking the drug in divided doses, with or immediately after meals, and by increasing the total daily dose very slowly; antacids taken 30-60 minutes before levodopa may also be beneficial. The vomiting has been attributed to stimulation of the chemoreceptor trigger zone located in the brainstem but outside the blood-brain barrier. Fortunately, tolerance to this emetic effect develops in many patients. Antiemetics such as phenothiazines should be avoided because they reduce the antiparkinsonism effects of levodopa and may exacerbate the disease. When levodopa is given in combination with carbidopa, adverse gastrointestinal effects are much less frequent and troublesome, occurring in less than 20% of cases, so that patients can tolerate proportionately higher doses. B. CARDIOVASCULAR EFFECTS A variety of cardiac arrhythmias have been described in patients receiving levodopa, including tachycardia, ventricular extrasystoles and, rarely, atrial fibrillation. This effect has been attributed to increased catecholamine formation peripherally. The incidence of such arrhythmias is low, even in the presence of established cardiac disease, and may be reduced still further if the levodopa is taken in combination with a peripheral decarboxylase inhibitor. Postural hypotension is common, but often asymptomatic, and tends to diminish with continuing treatment. Hypertension may also occur, especially in the presence of nonselective monoamine oxidase inhibitors or sympathomimetics or when massive doses of levodopa are being taken. C. DYSKINESIAS Dyskinesias occur in up to 80% of patients receiving levodopa therapy for long periods. The form and nature of dopa dyskinesias vary widely among patients but tend to remain constant in character in individual patients. Choreoathetosis of the face and distal extremities is the most common presentation. The development of dyskinesias is dose-related, but there is considerable individual variation in the dose required to produce them. D. BEHAVIORAL EFFECTS A wide variety of adverse mental effects have been reported, including depression, anxiety, agitation, insomnia, somnolence, confusion, delusions, hallucinations, nightmares, euphoria, and other changes in mood or personality. Such adverse effects are more common in patients taking levodopa in combination with a decarboxylase inhibitor rather than levodopa alone, presumably because higher levels are reached in the brain. They may be precipitated by intercurrent illness or operation. It may be

necessary to reduce or withdraw the medication.Several atypical antipsychotic agents (clozapine,olanzapine,quetiapine,and risperidone;see Chapter 29)are now available and may be particularly helpful in counteracting the behavioral complications of levodopa. E.FLUCTUATIONS IN RESPONSE Certain fluctuations in clinical response to levodopa occur with increasing frequency as treatment continues.In some patients,these fluctuations relate to the timing of levodopa intake,and they are then referred to as wearing-off reactions or end-of-dose akinesia.In other instances,fluctuations in clinical state are unrelated to the timing of doses (on-off phenomenon).In the on-off phenomenon,off-periods of marked akinesia alternate over the course of a few hours with on-periods of improved mobility but often marked dyskinesia.The phenomenon is most likely to occur in patients who responded well to treatment initially.The exact mechanism is unknown. For patients with severe off-periods who are unresponsive to other measures, subcutaneously injected apomorphine may provide temporary benefit. F.MISCELLANEOUS ADVERSE EFFECTS Mydriasis may occur and may precipitate an attack of acute glaucoma in some patients.Other reported but rare adverse effects include various blood dyscrasias;a positive Coombs test with evidence of hemolysis;hot flushes;aggravation or precipitation of gout;abnormalities of smell or taste;brownish discoloration of saliva, urine,or vaginal secretions;priapism;and mildusually transientelevations of blood urea nitrogen and of serum transaminases,alkaline phosphatase,and bilirubin. Drug Holidays A drug holiday (discontinuance of the drug for 3-21 days)may temporarily improve responsiveness to levodopa and alleviate some of its adverse effects but is usually of little help in the management of the on-off phenomenon.Furthermore,a drug holiday carries the risks of aspiration pneumonia,venous thrombosis,pulmonary embolism, and depression resulting from the immobility accompanying severe parkinsonism.For these reasons and because of the temporary nature of any benefit,drug holidays are no longer recommended. Drug Interactions Pharmacologic doses of pyridoxine (vitamin B6)enhance the extracerebral metabolism of levodopa and may therefore prevent its therapeutic effect unless a peripheral decarboxylase inhibitor is also taken.Levodopa should not be given to patients taking monoamine oxidase A inhibitors or within 2 weeks of their discontinuance,because such a combination can lead to hypertensive crises. Contraindications
necessary to reduce or withdraw the medication. Several atypical antipsychotic agents (clozapine, olanzapine, quetiapine, and risperidone; see Chapter 29) are now available and may be particularly helpful in counteracting the behavioral complications of levodopa. E. FLUCTUATIONS IN RESPONSE Certain fluctuations in clinical response to levodopa occur with increasing frequency as treatment continues. In some patients, these fluctuations relate to the timing of levodopa intake, and they are then referred to as wearing-off reactions or end-of-dose akinesia. In other instances, fluctuations in clinical state are unrelated to the timing of doses (on-off phenomenon). In the on-off phenomenon, off-periods of marked akinesia alternate over the course of a few hours with on-periods of improved mobility but often marked dyskinesia. The phenomenon is most likely to occur in patients who responded well to treatment initially. The exact mechanism is unknown. For patients with severe off-periods who are unresponsive to other measures, subcutaneously injected apomorphine may provide temporary benefit. F. MISCELLANEOUS ADVERSE EFFECTS Mydriasis may occur and may precipitate an attack of acute glaucoma in some patients. Other reported but rare adverse effects include various blood dyscrasias; a positive Coombs test with evidence of hemolysis; hot flushes; aggravation or precipitation of gout; abnormalities of smell or taste; brownish discoloration of saliva, urine, or vaginal secretions; priapism; and mild¾usually transient¾elevations of blood urea nitrogen and of serum transaminases, alkaline phosphatase, and bilirubin. Drug Holidays A drug holiday (discontinuance of the drug for 3-21 days) may temporarily improve responsiveness to levodopa and alleviate some of its adverse effects but is usually of little help in the management of the on-off phenomenon. Furthermore, a drug holiday carries the risks of aspiration pneumonia, venous thrombosis, pulmonary embolism, and depression resulting from the immobility accompanying severe parkinsonism. For these reasons and because of the temporary nature of any benefit, drug holidays are no longer recommended. Drug Interactions Pharmacologic doses of pyridoxine (vitamin B6) enhance the extracerebral metabolism of levodopa and may therefore prevent its therapeutic effect unless a peripheral decarboxylase inhibitor is also taken. Levodopa should not be given to patients taking monoamine oxidase A inhibitors or within 2 weeks of their discontinuance, because such a combination can lead to hypertensive crises. Contraindications