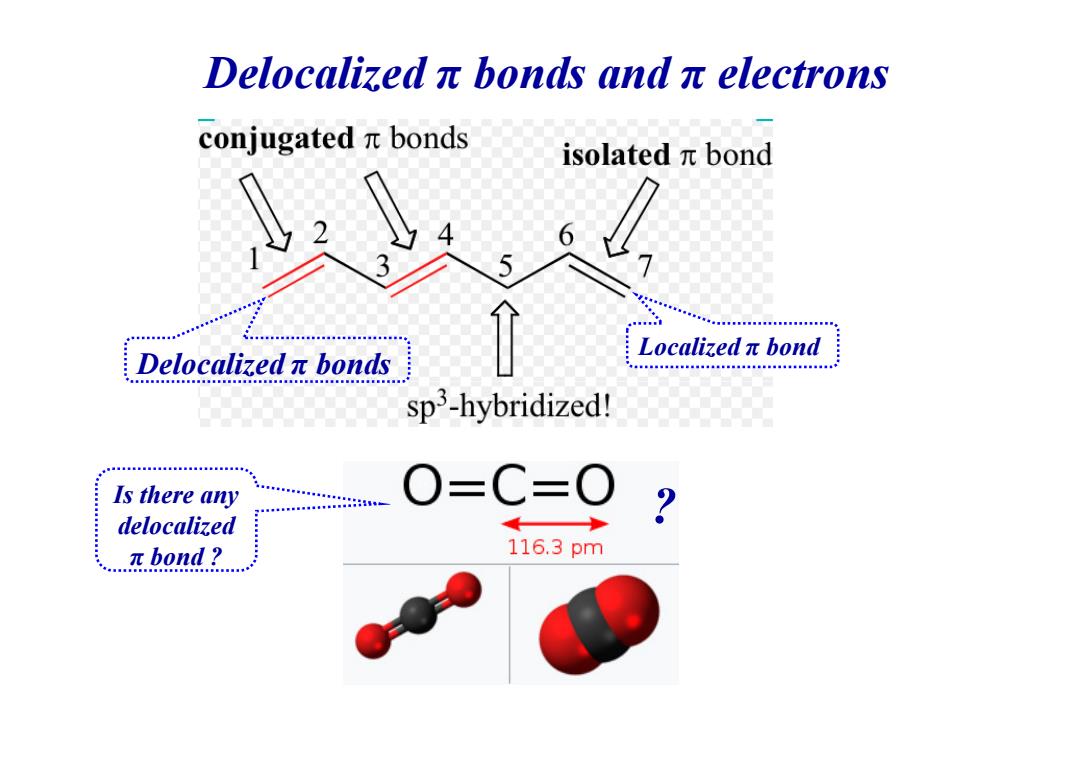
Delocalizedπbonds andπelectrons conjugatedπbonds isolatedπbond 6 Localizedπbond Delocalizedπbonds sp3-hybridized! 用目国图图名a Is there any O=C=0 delocalized πbond? 116.3pm
Delocalized π bonds and π electrons Delocalized π bonds Localized π bond Is there any delocalized π bond ? ?
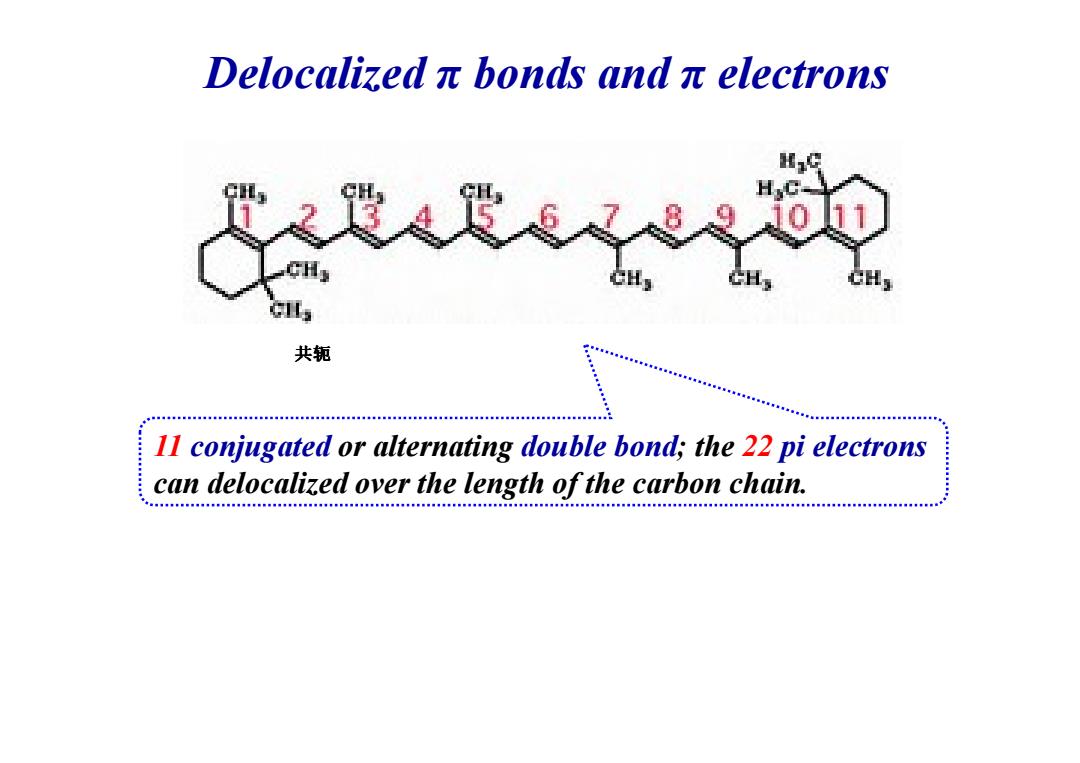
Delocalizedπbonds andπelectrons H 共轭 11 conjugated or alternating double bond;the 22 pi electrons can delocalized over the length of the carbon chain
Delocalized π bonds and π electrons 共轭 11 conjugated or alternating double bond; the 22 pi electrons can delocalized over the length of the carbon chain
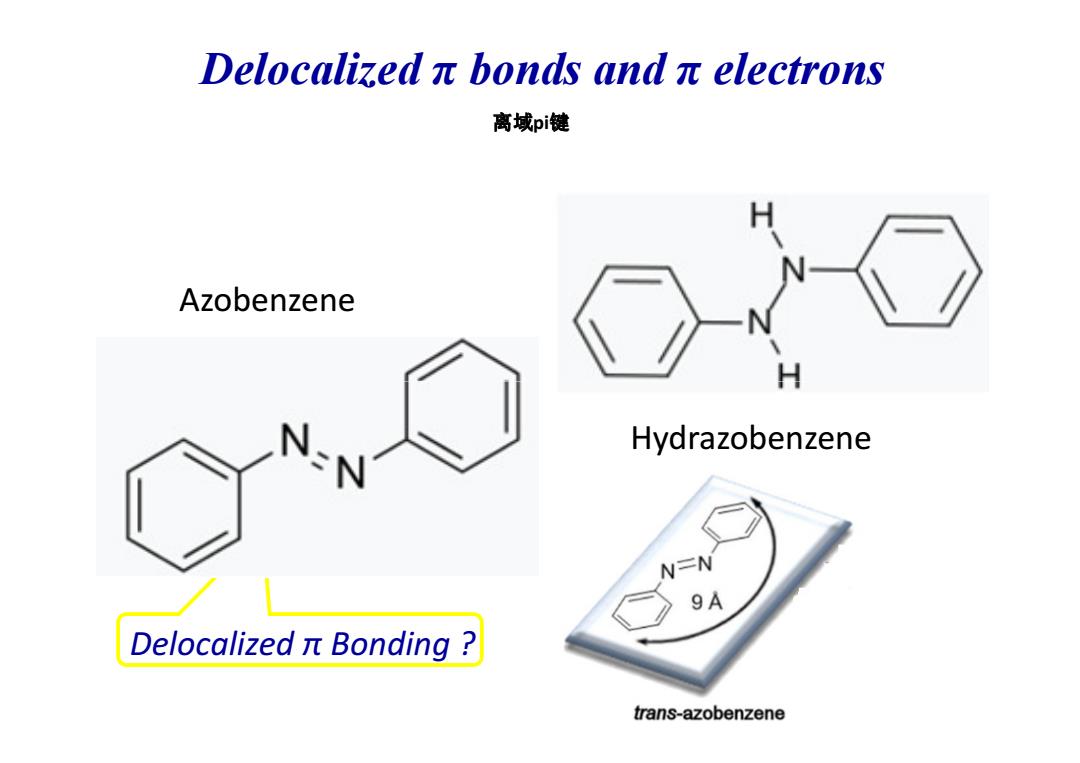
Delocalizedπbonds andπelectrons 离域pi键 Azobenzene H Hydrazobenzene N=N 9A Delocalized t Bonding trans-azobenzene
Azobenzene 离域pi键 Delocalized π bonds and π electrons Delocalized π Bonding ? Hydrazobenzene

Questions in your team work reports 4.Why does delocalization make the HOMO-LUMO gap smaller? (G.7,4,9,12,13,15,17) 5.If a molecule has several atoms with different maximum absorbable wavelength,do we say it has many maximum absorbable wavelength,or we just consider the largest one (G.7) 6.Does the electron inside a molecule only absorb the light which can be used to cover the HOMO-LUMO gap?(G.11) 7. Can the electrons in an organic molecule be excited only by the light of certain wavelength or all the wavelengths in the visible range?(G.7) 8. Will the number of excited electrons do any different to the color? (G.7) 9.What's the exact correlation between a molecule's color and the light it absorbs,resulting from its HOMO-LUMO gap?(G.11) 10.Can the relationship between HOMO-LUMO gap and absorbed light be quantized?(G.8)
4. Why does delocalization make the HOMO-LUMO gap smaller? (G. 7, 4, 9, 12, 13, 15, 17) 5. If a molecule has several atoms with different maximum absorbable wavelength, do we say it has many maximum absorbable wavelength, or we just consider the largest one ? (G. 7) 6. Does the electron inside a molecule only absorb the light which can be used to cover the HOMO-LUMO gap? (G. 11) 7. Can the electrons in an organic molecule be excited only by the Questions in your team work reports : light of certain wavelength or all the wavelengths in the visible range? (G. 7) 8. Will the number of excited electrons do any different to the color? (G. 7) 9. What’s the exact correlation between a molecule’s color and the light it absorbs, resulting from its HOMO-LUMO gap? (G. 11) 10. Can the relationship between HOMO-LUMO gap and absorbed light be quantized? (G. 8)