
Purpose (II) s.J.T.0. Phase Transformation and Applications The science of thermodynamics is concerned with heat and work.and transformations between the two. It is based on two laws of nature,the first and second laws of thermodynamics. By logical reasoning and skillful manipulation of these laws,it is possible to correlate many of the properties of materials and to gain insight into the many chemical and physical changes that materials undergo. SJTU Thermodynamics of Materials Spring 2009 ©X.J.Jin Lecture 1 First law I 6/38
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2009 © X. J. Jin Lecture 1 First law I 6/38 Purpose (II) The science of thermodynamics is concerned with heat and work, and transformations between the two. It is based on two laws of nature, the first and second laws of thermodynamics. By logical reasoning and skillful manipulation of these laws, it is possible to correlate many of the properties of materials and to gain insight into the many chemical and physical changes that materials undergo

Contents of the course S.J.T.0. Phase Transformation and Applications 热力学研究热功转换的方向、限度以及能量的衡算。 ·0,1st,2nd,3 rd laws Property Relationships e Equilibrium Chemical Equilibrium Electrochemistry ·Solution 。 Phase rule 。 Phase diagrams ● Statistical Thermodynamics Surfaces and Interfaces SJTU Thermodynamics of Materials Spring 2009 ©X.J.Jin Lecture 1 First law I 7/38
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2009 © X. J. Jin Lecture 1 First law I 7/38 Contents of the course • 0, 1st, 2nd, 3rd laws • Property Relationships • Equilibrium • Chemical Equilibrium • Electrochemistry • Solution • Phase rule • Phase diagrams • Statistical Thermodynamics • Surfaces and Interfaces 热力学研究热功转换的方向、限度以及能量的衡算

How to learn? S.J.T.U. Phase Transformation and Applications 。Reading ·Discussion 。Exercise God helps those who help themselves! SJTU Thermodynamics of Materials Spring2009©X.J.Jin Lecture 1 First law I 8/38
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2009 © X. J. Jin Lecture 1 First law I 8/38 How to learn? • Reading • Discussion • Exercise God helps those who help themselves!
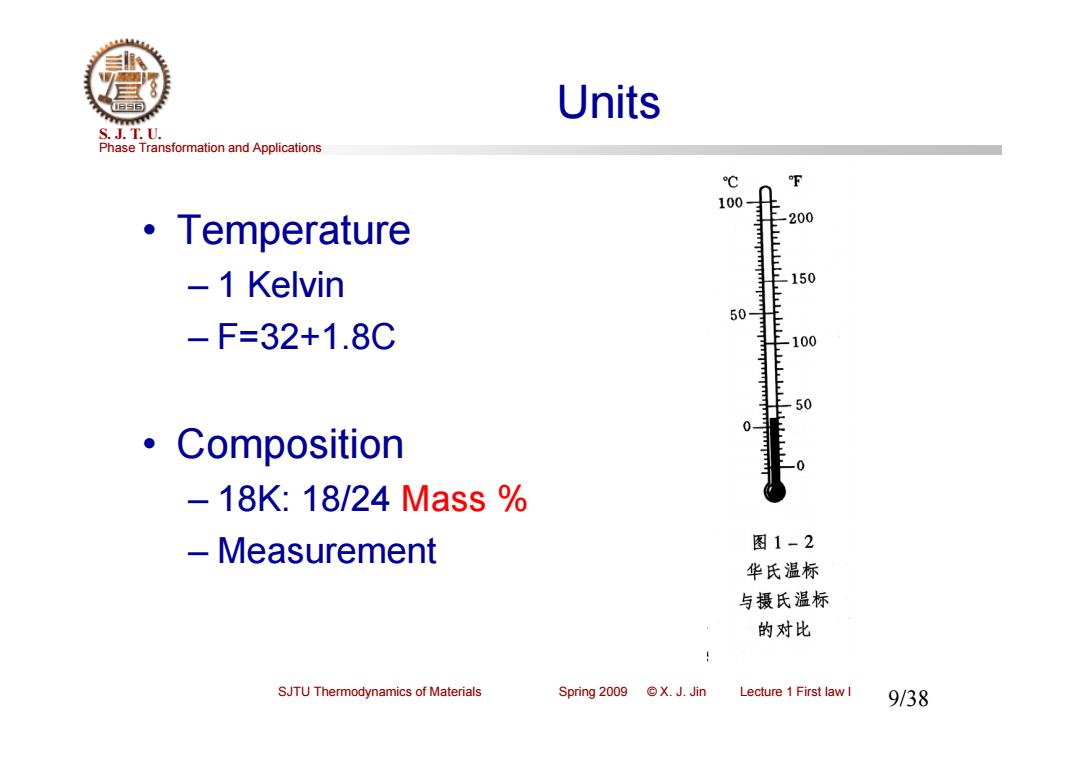
Units S.J.T.0. Phase Transformation and Applications ℃ 100 ·Temperature -200 -1 Kelvin 150 50- -F=32+1.8C -100 50 0 ·Composition -18K:18/24Mass% -Measurement 图1-2 华氏温标 与摄氏温标 的对比 SJTU Thermodynamics of Materials Spring 2009 ©X.J.Jin Lecture 1 First law I 9/38
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2009 © X. J. Jin Lecture 1 First law I 9/38 Units • Temperature – 1 Kelvin – F=32+1.8C • Composition – 18K: 18/24 Mass % – Measurement
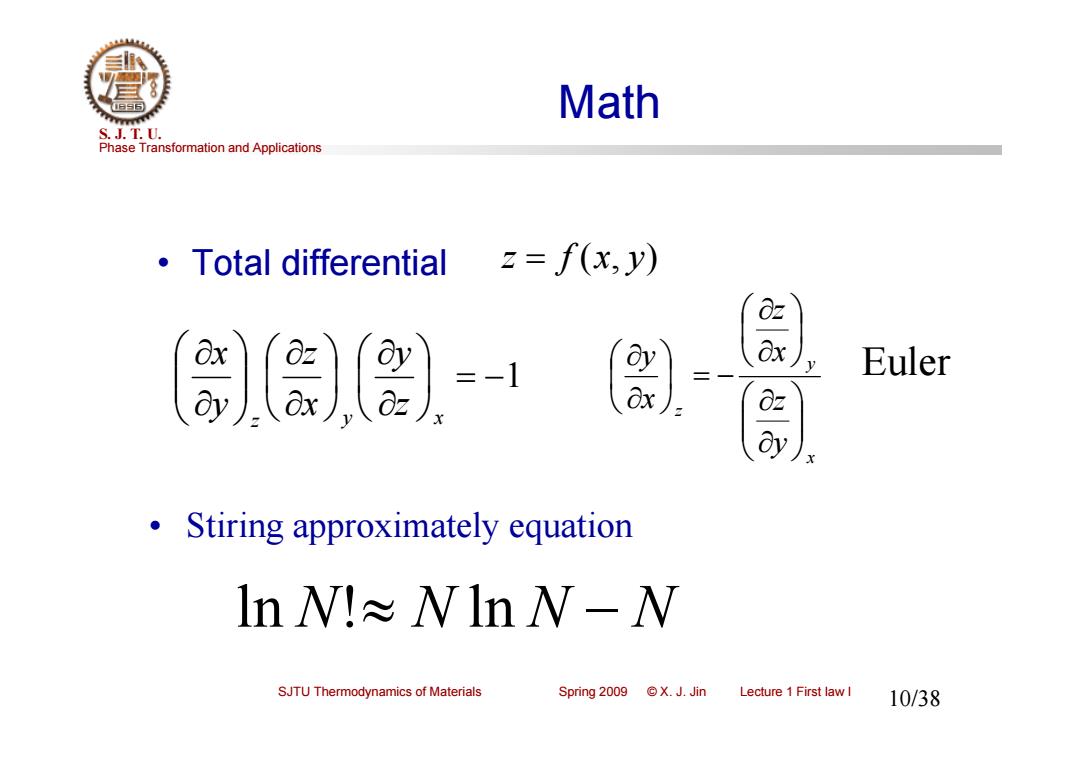
Math S.J.T.0. Phase Transformation and Applications ·Total differential 2=f(x,y) 0 a), Euler Stiring approximately equation lnW!≈WlnW-W SJTU Thermodynamics of Materials Spring 2009 ©X.J.Jin Lecture 1 First law I 10/38
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2009 © X. J. Jin Lecture 1 First law I 10/38 Math • Total differential ln N! N ln N N • Stiring approximately equation 1 z y x zy xz yx x y z y z x z x y Euler z f (x, y)