
Chapter 7.Solution Il Outline Nonideal Solutions非理想溶液 Gibbs-Duhem Relation吉布斯-杜海姆 Dilute solution稀溶液 Colligative properties依数性 Regular Solution规则溶液 Osmotic pressure(渗透压) Nov 11th,2016 pengzhang2010@sjtu.edu.cn 3/12/2022 Chapt 9:Solution
Outline Nonideal Solutions 非理想溶液 Gibbs-Duhem Relation 吉布斯-杜海姆 Dilute solution 稀溶液 Colligative properties 依数性 Regular Solution 规则溶液 Osmotic pressure (渗透压) Chapter 7. Solution II Nov 11th , 2016 pengzhang2010@sjtu.edu.cn 3/12/2022 Chapt 9: Solution 1

6.10 Lithium-chlorine batteries are being considered as the energy storage system for electric vehicles,even though they have to be operated at elevated temperatures.The reactants in the battery are pure lithium (Li)at one electrode and gaseous chlorine(Cl2)at the other.Assume that the electrolyte is pure lithium chlorine(LiCl).To charge the battery,a voltage is applied to the cell and the lithium chlorine is electrolyzed to form metallic lithium and gaseous chlorine(stored in a special way outside the cell).Upon discharge,the metallic lithium and gaseous chlorine react electrochemically to produce lithium chlorine. discharge Li (lithium)+1/2 Cl2(gas.)=Licl (liquid) charge (a)Estimate the open circuit voltage at 1000 K if the electrolyte is pure LiCl. (b)How many grams of lithium must the battery contain for each kilowatt-hour of energy delivered (Assume that the energy is delivered at the open circuit voltage.) (c)If the discharge rate is one kilowatt(at the open circuit voltage),at what rate must heat be added or removed to keep the temperature constant? (d)If the electrolyte is a solution containing 20%lithium chloride in other salts,and the solution is assumed to be ideal,will the open circuit voltage be different from the one determined in part a? (e)Will the answer to part c be changed? DATA Molecular weights: Lithium =6.94 g/mol Chlorine =35.45 g/mol For LiCl AG=-92,000+11T (cal/mol) 3/12/2022 Chapt 9:Solution 2
3/12/2022 Chapt 9: Solution 2
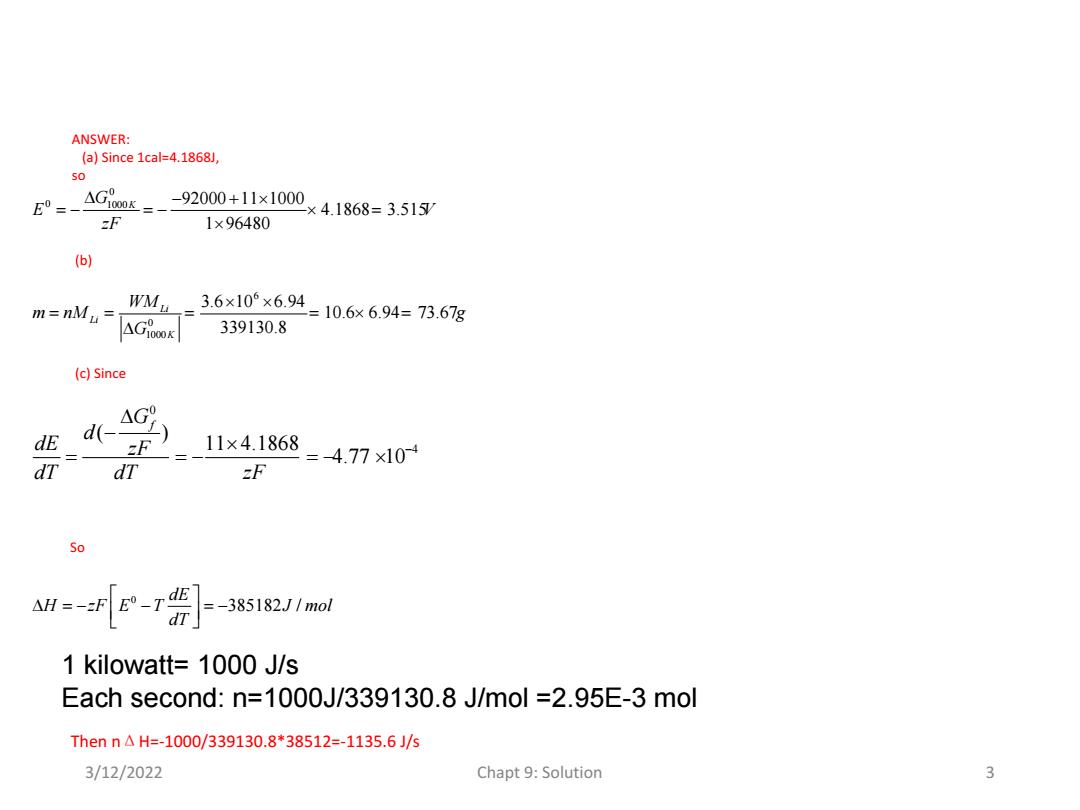
ANSWER: (a)Since 1cal=4.1868J, 50 GK= -92000+11×1000 ×4.1868=3.515/ zF 1×96480 (b) m=nMu= WM-3.6×10×6.94=10.6x6.94=73.67g AG0O 339130.8 (c)Since 4G9 dE d(- F 11×4.1868 dT dT zF =4.77×10 So =-f[E-1号]-385121mal 1 kilowatt=1000 J/s Each second:n=1000J/339130.8 J/mol =2.95E-3 mol Then n△H=-1000/339130.8*38512=-1135.6J/5 3/12/2022 Chapt 9:Solution 3
0 0 1000 92000 11 1000 4.1868 3.515 1 96480 G K E V zF 6 0 1000 3.6 10 6.94 10.6 6.94 73.67 339130.8 Li Li K WM m nM g G 0 4 ( ) 11 4.1868 4.77 10 Gf d dE zF dT dT zF ANSWER: (a) Since 1cal=4.1868J, so (b) (c) Since So 0 385182 / dE H zF E T J mol dT Then nΔH=-1000/339130.8*38512=-1135.6 J/s 1 kilowatt= 1000 J/s Each second: n=1000J/339130.8 J/mol =2.95E-3 mol 3/12/2022 Chapt 9: Solution 3
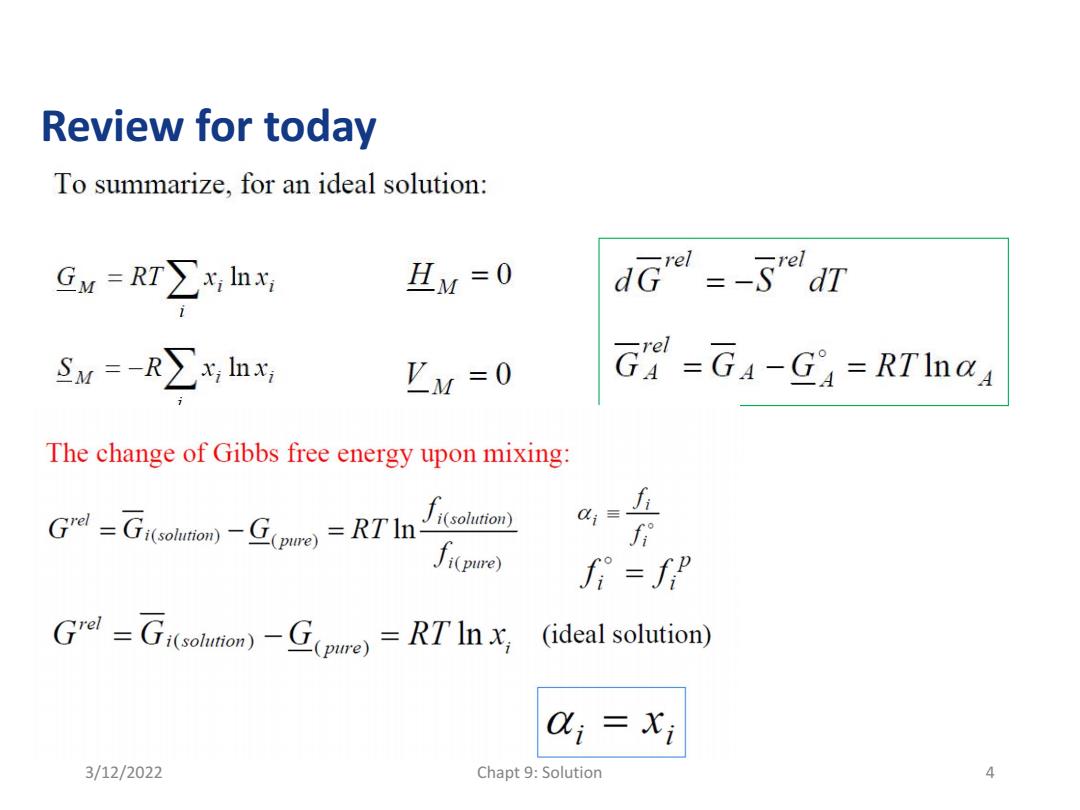
Review for today To summarize,for an ideal solution: Gw=RT∑x,h HM=0 dGr=-SrdT Su=-R∑xlh 'M=0 G=GA-Ga=RTInal The change of Gibbs free energy upon mixing: sot) f G=Giobuio)-G(pure)=RTIn Ji(pue) Grol =Gi(solumon)-G(pIre)=RT'In x (ideal solution) Qi =xi 3/12/2022 Chapt 9:Solution 4
Review for today 3/12/2022 Chapt 9: Solution 4
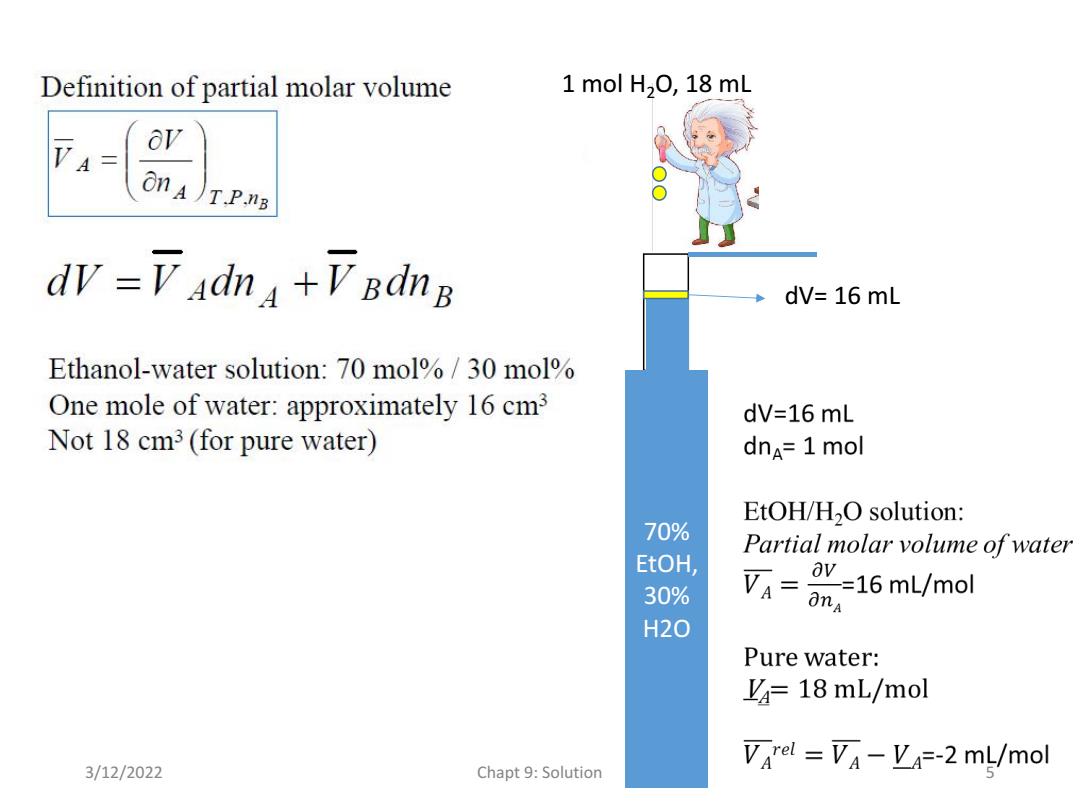
Definition of partial molar volume 1 mol H2O,18 mL ov on A)T.P.nB dv =V adna +V BdnB dV=16 mL Ethanol-water solution:70 mol%/30 mol One mole of water:approximately 16 cm3 dV=16 mL Not 18 cm3(for pure water) dnA=1 mol EtOH/H2O solution: 70% Partial molar volume of water EtOH, 30% av=16mL/mol anA H20 Pure water: L=18 mL/mol Varel Va-Va=-2 mL/mol 3/12/2022 Chapt 9:Solution
70% EtOH, 30% H2O 1 mol H2O, 18 mL dV= 16 mL 3/12/2022 Chapt 9: Solution 5